ABSTRACT: Diabetes is often associated with short-term and long-term complications, including diabetic kidney disease, diabetic neuropathy, and diabetic retinopathy. Not only are these complications a burden to the U.S. economy but they can have a significant impact on the patient. Tighter glycemic control, management of hypertension, and lifestyle changes have an overall benefit in managing all diabetes complications. This article discusses the pharmacologic interventions used in the management of diabetic neuropathy, diabetic kidney disease, diabetic retinopathy, and foot complications.
Uncontrolled diabetes can lead to a host of short- and long-term pathophysiological effects, including microvascular complications (neuropathy, retinopathy, and diabetic kidney disease), cardiovascular damage, gastrointestinal complications, increased risk of infection, and depression.1 Since diabetes complications can significantly impact a patient’s quality of life, pharmacists should look out for the symptoms and advise on the relevant care pathways.
It is estimated that around 10.5% of the U.S. population (34.2 million individuals) have diabetes.2 The total direct and indirect estimated costs of diagnosed diabetes in the U.S. increased from $188 billion in 2012 to $327 billion in 2017.2 That year, diabetes was the leading cause of end-stage kidney disease, and it currently affects about 20% of diabetes patients.3 Diabetic retinopathy is seen in nearly 50% of patients with diabetes and is currently the leading cause of new cases of blindness among adults aged 18 to 64 years.2,4
Overall, both better glycemic control and lifestyle modification work to reduce all diabetes complications. The prevention and management of diabetes complications through tighter glycemic control has been demonstrated in a number of trials. These include the Diabetes Control and Complications Trial, the Epidemiology of Diabetes Interventions and Complications study, the UK Prospective Diabetes Study, the Kumamoto Study, the Actions to Control Cardiovascular Risk in Diabetes Study, and the Fenofibrate Intervention and Event Lowering in Diabetes study.2,5-8
Diabetic Kidney Disease
Diabetic kidney disease (DKD), previously known as diabetic nephropathy, is characterized by persistent albuminuria, a reduced glomerular filtration rate (GFR), and elevated blood pressure. This leads to an increase in cardiovascular events and their related mortality.9
A comprehensive treatment and management plan for DKD consists of effective screening of patients, diet and lifestyle modification, and correction of hyperglycemia, hypertension and/or dyslipidemia, as well as subspeciality referral.3
Lifestyle modification typically includes moderation of alcohol intake, smoking cessation, increasing physical activity, reducing weight, and dietary modification. As far as diet modification goes, there is evidence that it prevents the progression of DKD. However, there is no consensus on which particular intervention is the most effective. Protein restriction to less than 0.8 g per kg per day has been known to slow the decline of the GFR and progression to end-stage renal disease (ESRD).3 Similarly, the Dietary Approaches to Stop Hypertension diet and the Mediterranean diet have proven beneficial. Individuals on these diets should consume no more than 2,300 mg per day of sodium, primarily through whole-grain carbohydrates, fiber, fresh fruits, fresh vegetables, and omega-3 and omega-9 fats. Sugar, saturated fats, and processed carbohydrates should be avoided.3
A range of drugs has been studied for their positive effects on renal outcomes. Both angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) delay and reduce the progression of DKD.3 It has been demonstrated that ACE inhibitors reduce the risk of new-onset microalbuminuria or macroalbuminuria in individuals with diabetes with or without hypertension.3 Furthermore, ACE inhibitors have been found to reduce the risk of death in patients with diabetes compared with placebo.3 TABLE 1 lists some of the newer drugs that have been used in the management of DKD.9
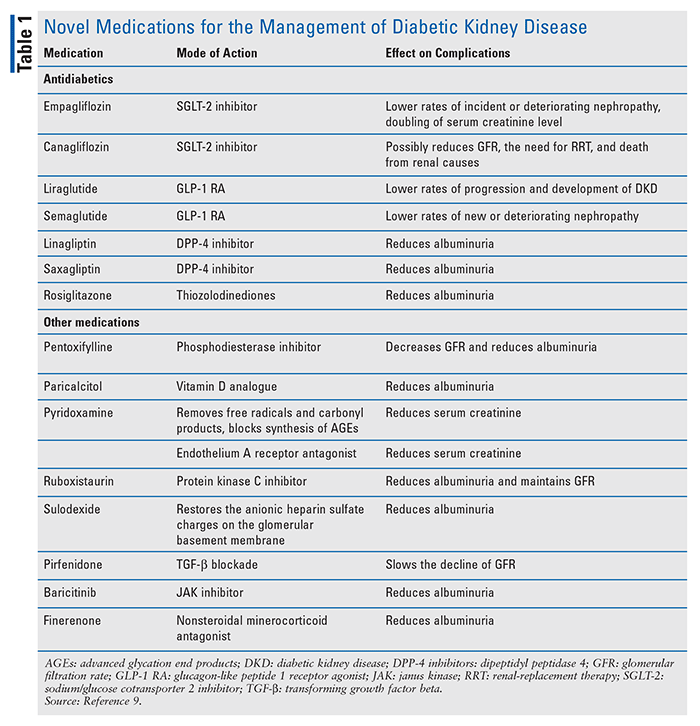
The Eighth Joint National Committee and the American Diabetes Association recommend that the target blood pressure for diabetics should be lower than 140/90 mmHg.10 The American College of Cardiology/American Heart Association guidelines recommend a target of lower than 130/80 mmHg. It is suggested that this should be achieved through lifestyle management and weight loss in overweight/obese patients, together with pharmacologic therapy if necessary.3 ACE inhibitors and ARBs are effective at lowering blood pressure and reducing the urinary albumin excretion rate for microalbuminuric patients with type 2 diabetes mellitus (T2DM).9
Patients with DKD often have an altered lipid metabolism, leading to increased LDL cholesterol (LDL-C) complex and increasing the risk of cardiovascular events in this population. Statin therapy primarily reduces cardiac events and may have a smaller renoprotective effect. Statins are recommended in diabetic patients older than age 21 years with a primary LDL-C higher than 190 mg/dL and in those aged 40 to 75 years with an LDL-C level between 70 and 189 mg/dL.9 The Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease trial concluded that atorvastatin has a more renoprotective effect in reducing proteinuria and renal events than rosuvastatin in patients with DKD who are on ACE inhibitors or ARB therapy.9 The doses of statins that are metabolized by the kidneys may need to be reduced in patients with a significantly decreased GFR. Atorvastatin does not need to be adjusted in these patients since it is not metabolized by the kidneys.3
Renal replacement therapy may be offered to patients with ESRD. This includes one of the following: peritoneal dialysis, hemodialysis, or renal transplantation.11
Diabetic Neuropathy
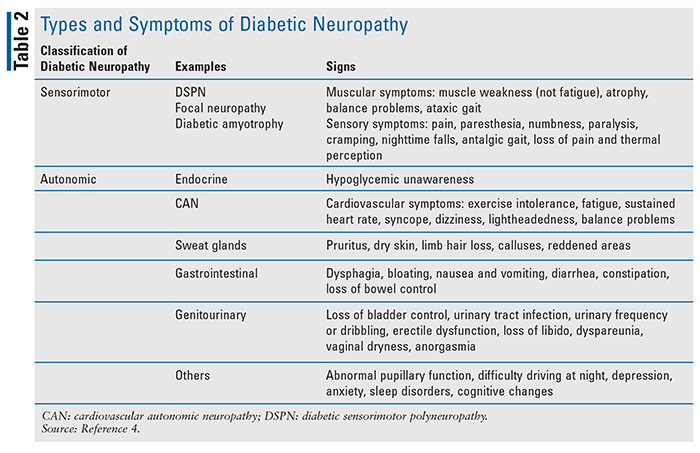
Diabetic neuropathies are common in all patients with T2DM, in those who have had type 1 diabetes mellitus (T1DM) longer than 5 years, and in patients with prediabetes.4 There are different types of neuropathies, affecting different parts of the nervous system. It is important to note that patients may develop more than one type of neuropathy.4 TABLE 2 lists some of these neuropathies. Peripheral nerve dysfunction in diabetics without any other underlying cause is classified as DSPN.12 Autonomic neuropathies affect the involuntary physiological functions, such as thermoregulation and sweat production. As a result, damage to the affected nerves causes loss of skin elasticity, dry skin, calluses, and fissuring. They include cardiovascular autonomic neuropathy (CAN) and gastrointestinal, genitourinary, and sudomotor dysfunction. In the case of CAN, oxygen bypasses capillary beds and oxygenated blood enters the venous system, resulting in the deprivation of oxygen and nutrients to tissues.13
Neuropathic or DSPN Pain Management
Specific strategies for managing neuropathic pain in diabetics is yet to be fully established. While there are many studies addressing overall neuropathic pain, only a few target DSPN. There is no consensus on the guidelines, but the guidelines issued by the American Academy of Neurology, American Academy of Physical Medicine and Rehabilitation, and the American Academy of Physical Medicine and Rehabilitation recommend the use of pregabalin as first-line therapy.14
The FDA-approved agents for the treatment of DSPN pain are limited to pregabalin, duloxetine, and extended-release tapentadol.12 Other agents that have been used off-label include anticonvulsants, the monoamine reuptake inhibitors, and opioids.15
Clinical trials conducted on the anticonvulsants pregabalin, gabapentin, and sodium valproate have shown mixed results in the reduction of DSPN pain.12 However, most guidelines still recommend the use of these drugs in the management of DSPN. Gabapentin is used off-label in doses of 900 mg/day initially. This dose can be increased gradually every 3 days up to a maximum of 1,800 to 3,600 mg/day.14
Pregabalin is started at 50 mg three times a day (150 mg/day) and can be increased to a maximum of 100 mg three times a day (300 mg/day) within 1 week based on efficacy and tolerability.16 Doses should also be adjusted in adults with reduced renal function or in older patients in whom side effects may be more severe. The most common side effects are dizziness, somnolence, dry mouth, edema, blurred vision, weight gain, and abnormal thinking.16
Monoamine reuptake inhibitors can be further divided into serotonin and norepinephrine reuptake inhibitors, tricyclic antidepressants, and selective serotonin reuptake inhibitors. Duloxetine was the first antidepressant to be approved specifically for the treatment of diabetic neuropathy.17 The recommended dose of duloxetine is 60 mg/day, but the dosage can be lowered if tolerability is a concern.18 The most common adverse effects seen in patients taking duloxetine are nausea, dry mouth, somnolence, fatigue, constipation, decreased appetite, and hyperhidrosis. CYP1A2 inhibitors should be avoided in patients taking duloxetine.18 CYPD26 inhibitors may reduce duloxetine concentrations. Other antidepressants with venlafaxine have shown effectiveness in doses of 150 to 225 mg/day. Amitriptyline, a tricyclic antidepressant, is one of the most often used, non–FDA-approved medications for DSPN pain.12 It is typically used in doses of 65 to 100 mg/day for at least 3 weeks.19
Tramadol and other opioids have shown some efficacy in the management of DSPN pain.12 However, due to the high risk of addiction and associated safety concerns, these agents are not recommended as first-line management of DSPN pain. When used, tapentadol ER should be initiated in opioid-naïve patients at a dose of 50 mg twice daily, titrated up by 50 mg twice daily every 3 days to a maximum daily dose of 500 mg/day.20 The most common side effects noted with tapentadol ER are nausea, constipation, dizziness, headache, and somnolence. The use of tapentadol is not recommended in patients with severe hepatic or renal impairment.20
Orthostatic Hypotension
Orthostatic hypotension associated with CAN is managed through pharmacologic and nonpharmacologic interventions.12 Nonpharmacologic modalities include physical activity and volume repletion with increased intake of fluids and salt.12 Pharmacologic management focuses on the use of sympathomimetic agents, such as midodrine and droxidopa.
Midodrine is FDA approved for the treatment of orthostatic hypotension but needs to be slowly titrated to efficacy.12 The recommended dose is 2.5 to 10 mg, three times daily, with the last dose being given no later than 6 PM.21 The most common side effects noted with the use of midodrine are supine and sitting hypertension, paresthesia and pruritus (mainly of the scalp), goosebumps, chills, urinary urge, urinary retention, and urinary frequency.22 Its use is contraindicated in patients with severe organic heart disease, acute renal disease, urinary retention, pheochromocytoma, thyrotoxicosis, or persistent and excessive supine hypertension.
Similarly, droxidopa is FDA approved for the treatment of neurogenic orthostatic hypotension.12 The recommended dose is 100 mg three times during the day titrated by 100 mg three times daily to efficacy (maximum dose 1,800 mg/day). The last dose should be given at least 3 hours prior to bedtime. Droxidopa is associated with headache, dizziness, nausea, hypertension, and fatigue.23
Gastroparesis
In some cases, gastroparesis may be managed by making dietary changes, such as eating multiple small meals and decreasing dietary fat and fiber intake.12 Pharmacists can advise patients on how to keep a food diary and thereby determine what types of food exacerbate symptoms. Generally, it is noted that liquid fats are better tolerated than solid fats. Fiber not only slows the rate of gastric emptying but can lead to the formation of bezoars. These are solid masses of partially or undigested material that can cause blockages in the gastrointestinal tract. Pharmacists should review patients’ drug profiles to ensure that they are not taking any medications that can slow gastrointestinal motility.24 These may include opioids, anticholinergics, tricyclic antidepressants, GLP-1 receptor agonists, pramlintide, and dipeptidyl peptidase 4 inhibitors.
Metoclopramide is the only drug that has FDA approval for the management of gastroparesis. However, since the evidence of its benefits are weak and it is associated with a risk of serious side effects (extrapyramidal symptoms), its use is not recommended for more than 5 days. The recommended dose for patients with acute and recurrent diabetic gastroparesis is 10 mg, 30 minutes before each meal and at bedtime (maximum of 40 mg/day). The most common side effects are restlessness, drowsiness, fatigue, and lassitude.25 Metoclopramide should not be given to patients with a history of tardive dyskinesia, those in whom stimulation of gastrointestinal motility might be dangerous, patients with pheochromocytoma, catecholamine-releasing paragangliomas, or epilepsy.25
Vision Complications
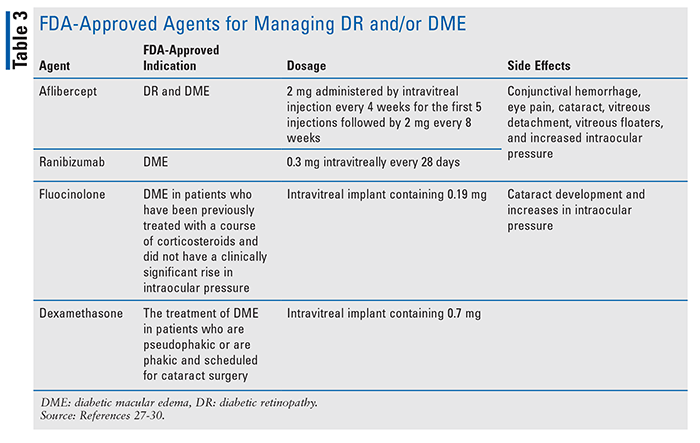
Diabetic retinopathy (DR) and diabetic macular edema (DME) are common complications associated with diabetes.26 Microvascular complications in diabetics can suddenly impact a patient’s visual acuity and eventually lead to blindness. DR is a major cause of blindness in the working adult population in the U.S.7 Traditionally, laser therapy has been used to treat patients with DR or DME. More recently, pharmacologic agents that control hyperglycemia, hypertension, and dyslipidemia, as well as ocular agents administered intravitreally, have been employed for treatment. Intravitreal therapy includes antivascular endothelial growth factor agents such as bevacizumab, aflibercept, ranibizumab, pegaptanib, and conbercept; corticosteroids such as dexamethasone, fluocinolone, and triamcinolone; and nonsteroidal anti-inflammatory agents such as diclofenac, nepafenac, and ketorolac.27-30
TABLE 3 lists the FDA-approved agents used in the management of diabetic vision complications.
Foot Complications
Patients with diabetes can develop various foot problems, including ulcers and foot infections. Foot complications in diabetics are primarily caused by peripheral neuropathy, peripheral arterial disease (PAD), and impaired immunity. PAD is an atherosclerotic occlusive disease of the lower extremities in which the blood supply to the lower limbs is reduced. As a result, the skin is more susceptible to minor trauma, wound healing is impaired, and the risk of infection increases. PAD can lead to necrosis or gangrene and the loss of a limb. It is the underlying cause of a third of foot ulcers and a significant factor in reulceration.31
Diabetic foot infections are responsible for more than 50% of nontraumatic lower extremity amputations. Around 85% of all lower extremity amputations in patients with diabetes are preceded by an ulcer. Pharmacists can reduce this by counseling patients on their daily foot care routine. It is important that patients inspect their feet daily for dry or cracking skin, fissures, plantar callus formation, and signs of infection between the toes and around the toenails. They should avoid the application of topical ointments to intertriginous areas.32 Footwear should fit properly, and new shoes that commonly cause ulceration should be broken in slowly.
Patients need to be advised to avoid potential sources of trauma such as walking barefoot, cutting nails incorrectly, and exposing their feet to hot objects or chemicals such as hydrogen peroxide, iodine, or astringents such as witch hazel.32 Pharmacists should encourage patients to have an annual foot checkup with their physicians. Owing to the high incidence of PAD and neuropathy, the classic signs of infection may not be present in diabetic foot ulcers. It is imperative that healthcare professionals are more mindful of subtle signs of infection, such as an increase in wound exudate, friable granulation tissue, malodor, and undermining of the wound.
Ulcers may be managed by offloading and dressings. Milder foot infections are often treated with oral antibiotics in the outpatient setting. They are caused by mild gram-positive cocci. Clindamycin, doxycycline, linezolid, minocycline, and trimethoprim/sulphamethoxazole are active against methicillin-resistant Staphylococcus aureus (MRSA). Amoxicillin/clavulanate, cefdinir, cephalexin, dicloxacillin, or levofloxacin can also be used against MRSA-negative gram-positive cocci. Moderate-to-severe infections typically require parenteral antibiotics in a hospital setting.32 These infections may be caused by gram-positive cocci, gram-negative rods, or anaerobes. Daptomycin, linezolid, tigecycline, and vancomycin are effective against MRSA organisms in moderate-to-severe infections. MRSA-negative moderate-to-severe infection may be managed by ampicillin/sulbactam, cefoxitin, ceftriaxone, clindamycin, ertapenem, imipenem/cilastin, moxifloxacin, piperacillin/tazobactam, or ticarcillin/clavulanate.32
Conclusion
Even with the recent developments in newer antidiabetic medications and other therapies to manage related complications, the burden of diabetes complications remains high. There is still a need for more effective therapies to improve patient outcomes. Until these become available, pharmacists should continue to remain vigilant when looking out for signs or symptoms of complications, help diabetics manage their glycemic levels, and direct patients to take appropriate measures to prevent the worsening of any developing complications.
REFERENCES
1. Nathan DM. DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: Overview. Diabetes Care. 2014;37(1):9-16.
2. CDC. National Diabetes Statistics Report, 2020. Atlanta, GA: CDC. U.S. Department of Health and Human Services; 2020.
3. McGrath K, Edi R. Diabetic kidney disease: diagnosis, treatment, and prevention. Am Fam Physician. 2019;99(12):751-759.
4. Aring AM, Jones DE, Falko JM. Evaluation and prevention of diabetic neuropathy. Am Fam Physician. 2005;71(11):2123-2128.
5. UKPDS Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
6. Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(suppl 2): B21-B29.
7. Mansour SE, Browning DJ, Wong K, et al. The evolving treatment of diabetic retinopathy. Clin Ophthalmol. 2020;14:653-678.
8. Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687-1697.
9. Lin YC, Chang YH, Yang SY, et al. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117(8):662-675.
10. Armstrong C. Joint National Committee. JNC8 guidelines for the management of hypertension in adults. Am Fam Physician. 2014;90(7):503-504.
11. Batuman V. Diabetic nephropathy treatment & management. Medscape. https://emedicine.medscape.com/article/238946-overview. Accessed August 19, 2020.
12. Ang L, Cowdin N, Mizokami-Stout K, Pop-Busui R. Update on the management of diabetic neuropathy. Diabetes Spectr. 2018;31(3):224-233.
13. Weaving L, Berrington R. The diabetic foot. Pharmaceutical Journal. 2017;suppl.
14. Medscape. Gabapentin. Online. Accessed October 14, 2020. https://reference.medscape.com/drug/neurontin-gralise-gabapentin-343011.
15. Quan D. Online. Diabetic neuropathy treatment & management. Medscape https://emedicine.medscape.com/article/1170337-treatment#showall. Accessed August 19, 2020.
16. Pfizer. Lyrica Prescribing Information. www.accessdata.fda.gov/drugsatfda_docs/label/2018/021446s035,022488s013lbl.pdf. August Accessed August 18, 2020.
17. Quann D. Diabetic neuropathy medication. Medscape. Online. Accessed October 14, 2020. https://emedicine.medscape.com/article/1170337-medication#6.
18. Eli Lilly and Company. Cymbalta Prescribing Information. Online. Accessed August 18, 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2010/022516lbl.pdf
19. Medscape. Amitriptyline. Online. Accessed October 14, 2020. https://reference.medscape.com/drug/levate-amitriptyline-342936.
20. Depomed, Inc. Nucynta ER Prescribing Information. Online. Accssed August 18, 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2016/200533s014lbl.pdf.
21. Medscape. Midodrine. Online. Accessed October 14, 2020. https://reference.medscape.com/drug/proamatine-orvaten-midodrine-342442.
22. Shire US Inc. ProAmatine Prescribing Information. Online. Accessed August 18, 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2017/019815s010lbl.pdf.
23. Chelsea Therapeutics, Inc. Northera Prescribing Information. Online. Accessed August 18, 2020.www.accessdata.fda.gov/drugsatfda_docs/label/2014/203202lbl.pdf.
24. Careyva B, Stello B. Diabetes mellitus: management of gastrointestinal complications. Am Fam Physician. 2016;94(12):980-986.
25. ANI Pharmaceuticals Inc. Reglan Prescribing Information. Online. Accessed August 19, 2020.www.accessdata.fda.gov/drugsatfda_docs/label/2017/017854s062lbl.pdf.
26. Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653-2664.
27. Regeneron Pharmaceuticals Inc. Eyelea Prescribing Information. Online. Accessed August 19, 2020.www.accessdata.fda.gov/drugsatfda_docs/label/2019/125387s061lbl.pdf.
28. Genentech, Inc. Lucentis Prescribing Information. Online. Accessed August 19, 2020.www.accessdata.fda.gov/drugsatfda_docs/label/2014/125156s105lbl.pdf.
29. Alimera Sciences, Inc. Iluvien Prescribing Information. Online. Accessed August 19, 2020.www.accessdata.fda.gov/drugsatfda_docs/label/2017/201923s002lbl.pdf.
30. Allergan Inc. Ozurdex Prescribing Information. Online. Accessed August 19, 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022315s009lbl.pdf.
31. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment. Diabetes Care. 2008;31(8):1679-1685.
32. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88(3):177-184.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





