US Pharm. 2023;48(2):59-60.
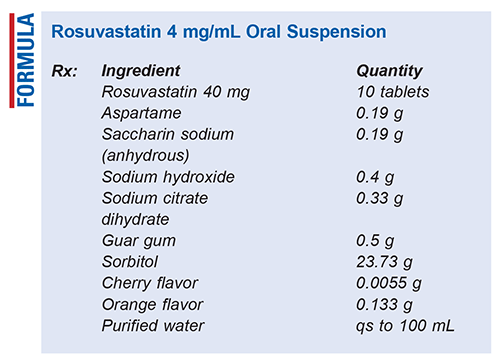
Method of Preparation: Calculate the amount of each ingredient required for the total amount to be prepared. Allow tablets to air-dry for a few minutes after removing the trademark imprint with a paper towel dampened with alcohol, USP. Triturate tablets to a fine powder using a mortar and pestle. Weigh out appropriate amounts of the saccharin sodium, sodium hydroxide, trisodium citrate, guar gum, cherry flavor, and orange flavor powders. Using consistent mixing and trituration, add these ingredients to the mortar, mixing continuously. Once the powders have been homogenously mixed and refined, weigh out appropriate amount of sorbitol and gradually add and mix in the mortar. Transfer in enough water to dissolve the powder in the mortar; stir until thoroughly dissolved. Transfer contents to a calibrated bottle. Bring final volume to 100 mL with water. Shake well until the powder is uniformly suspended.
Use: Rosuvastatin oral suspension has been used to lower serum LDL cholesterol in pediatric and adult patients.
Packaging: Package in a tight, light-resistant container. Store at controlled room temperature.
Labeling: Keep out of reach of children. Keep refrigerated. Protect from light. Shake well. Discard after ____ [time period].
Stability: The USP default beyond-use date for nonpreserved aqueous oral liquids is 14 days when stored in a refrigerator.1
Quality Control: Quality-control assessments include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).2
Discussion: Rosuvastatin (Crestor, (C22H27FN3O6S)2Ca, MW 1001.14) is a white, amorphous powder that is sparingly soluble in water, methanol, and ethanol. With a pH of 7.0, rosuvastatin calcium is a hydrophilic compound with an octanol/water partition coefficient of 0.13. During extemporaneous preparation, each tablet contains rosuvastatin 5 mg, 10 mg, 20 mg, or 40 mg. Each tablet contains these inactive ingredients: microcrystalline cellulose, lactose monohydrate, tribasic calcium phosphate, crospovidone, magnesium stearate, hypromellose, triacetin, titanium dioxide USP, yellow ferric oxide, and red ferric oxide. Rosuvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, reduces the body’s production of certain fatty substances, including cholesterol. It is used in conjunction with a healthy diet to lower high cholesterol and triglycerides and increase HDL cholesterol, as well as to slow progression of atherosclerosis.3
Aspartame (C14H18N2O, MW 294.31) is a sweet-tasting, off-white, almost odorless, crystalline powder. The pH in a 0.8% w/v aqueous solution is 4.5 to 6.0. Aspartame is slightly soluble in 95% ethanol and sparingly soluble in water. Higher temperatures and a more acidic pH environment increase its solubility. Aspartame is a sweetening agent used to enhance flavors and mask unpleasant tastes.4
Saccharin sodium anhydrous (C7H4NNaO3S, MW 205.16) is a white, odorless, efflorescent, crystalline powder with a strong, sweet taste and a metallic aftertaste. The pH in a 10% w/v aqueous solution is 6.6. Saccharin sodium anhydrous has varying solubility in different solvent solutions (e.g., ethanol, propylene glycol); it is more soluble than saccharin in water and is insoluble in propan-2-ol. Significant decomposition can occur after saccharin sodium anhydrous is exposed to high temperatures (125°C) and low pH (pH 2) for more than an hour. It is also used as a sweetener.5
Sodium hydroxide (NaOH, MW 40.00) comes in a variety of forms (pellets, flakes, sticks, etc.) and is white-colored. When exposed to air, it can become very deliquescent and quickly absorb carbon dioxide and water. The pH in a 5% w/w aqueous solution is ~14. Sodium hydroxide has a melting point of 318°C; it is practically insoluble in ether but soluble in glycerin. Sodium hydroxide is an alkalizing and buffering agent that isused to adjust the pH of solutions or to react with weak acids to form salts.6
Sodium citrate dihydrate (C6H5Na3O7 • 2H2O, MW 294.10) is a white, odorless powder. In a moist environment, the compound is slightly deliquescent; in warm air, it is efflorescent. When placed in a 5% w/v aqueous solution, sodium citrate dihydrate has a pH range of 7.0 to 9.0, and it converts to anhydrous form when heated to 150°C. It is practically insoluble in 95% ethanol and soluble in water 1:1.5. Sodium citrate dihydrate is used to adjust the pH of solutions, and it can act as a sequestering agent.7
Guar gum ((C6H12O6)n, MW ~220,000) is a tasteless, odorless, white to yellowish-white powder that consists of a high-MW hydrocolloidal polysaccharide. It has a pH of 5.0-7.0 in a 1% w/v aqueous dispersion. Guar gum is practically insoluble in organic solvents; in water, it forms a highly viscous, thixotropic solution. Its optimal hydration rate occurs at a pH of 7.5 to 9.0. Guar gum is a binder and disintegrant in solid products and a suspending, thickening, and stabilizing agent in oral and topical forms.8
Sorbitol (C6H14O6, MW 182.17) is a sweet-tasting, odorless, white, crystalline powder. It comes in a variety of forms, including granules, flakes, and pellets. Sorbitol has a pH of 4.5 to 7.0 in a 10% w/v aqueous solution. It is practically insoluble in chloroform and ether, slightly soluble in methanol, and 1 in 0.5 soluble in water. Sorbitol will form water-soluble chelates with metal ions in highly acidic conditions. Sorbitol is used in liquid preparations as a vehicle for sugar-free compounds and as a stabilizer for drug suspensions.9
Purified water is water that has been filtered using distillation, ion exchange, reverse osmosis, or another suitable process. It is miscible with most polar solvents and is chemically stable in all physical states.10
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; September 2022.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. Crestor (rosuvastatin calcium) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2022.
4. Wang H. Aspartame. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of Pharmaceutical Excipients. 5th ed. Washington, DC: American Pharmaceutical Association; 2006:53-55.
5. Owen SC. Saccharin sodium. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of Pharmaceutical Excipients. 5th ed. Washington, DC: American Pharmaceutical Association; 2006:641-643.
6. Kibbe AH. Sodium hydroxide. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of Pharmaceutical Excipients. 5th ed. Washington, DC: American Pharmaceutical Association; 2006:683-684.
7. Amidon GE. Sodium citrate dihydrate. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of Pharmaceutical Excipients. 5th ed. Washington, DC: American Pharmaceutical Association; 2006:675-677.
8. Kibbe AH. Guar gum. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of Pharmaceutical Excipients. 5th ed. Washington, DC: American Pharmaceutical Association; 2006:315-317.
9. Owen SC. Sorbitol. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of Pharmaceutical Excipients. 5th ed. Washington, DC: American Pharmaceutical Association; 2006:718-721.
10. Dubash D, Shah U. Water. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. Washington, DC: American Pharmaceutical Association; 2017:1012-1016.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





