US Pharm. 2020;45(2):18-22.
ABSTRACT: Patients with diabetes are at higher risk for heart failure compared with healthy patients. Drugs in the newest class of medications for type 2 diabetes—sodium-glucose cotransporter 2 (SGLT2) inhibitors—reduce blood glucose via renal glucose reabsorption, resulting in urinary excretion of glucose. SGLT2 inhibitor therapy has resulted in significant reductions in glycosylated hemoglobin A1c levels, weight, and blood pressure. Cardiovascular outcomes trials have also demonstrated positive outcomes in terms of heart failure. Although SGLT2 inhibitors may be a beneficial adjunct to guideline-directed medical therapy for diabetes patients with heart failure, the pill burden and patient cost would be greater. Pharmacists are well positioned to discuss the advantages and disadvantages of these agents with prescribers and patients.
Patients receiving a diagnosis of diabetes mellitus (DM) initially begin metformin therapy and comprehensive weight management involving diet and exercise.1 Metformin, which has minimal side effects, has proven benefits for glycosylated hemoglobin A1C (HbA1c), weight, and cardiovascular (CV) mortality.1 If DM remains uncontrolled despite metformin and lifestyle management, additional therapy is needed. The American College of Cardiology’s atherosclerotic CV disease (ASCVD) risk estimator is used to measure an individual patient’s risk of developing ASCVD.2 If the patient has established ASCVD or chronic kidney disease (CKD), a glucagon-like peptide-1 receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor could be initiated.1 If heart failure (HF) or CKD predominates, SGLT2 inhibitor therapy should be initiated.
Patients with DM are at higher risk for HF.3 DM causes hyperglycemia, insulin resistance, and hyperinsulinemia, which trigger vascular smooth-muscle inflammation and dyslipidemia. This cascade promotes faster development of coronary artery disease, which is the leading cause of myocardial dysfunction.3 Additionally, DM patients have higher left ventricular mass, wall thickness, prolonged pre-ejection periods and shortened ejection times, and reduced systolic and diastolic functions, all of which can lead to HF.4 Risk factors associated with HF in DM patients include higher BMI, poor glycemic control, insulin use, long duration of DM, and microalbuminuria.5
SGLT2 inhibitors are the most recent drug class approved by the FDA for treating type 2 DM (T2DM). They work by inhibiting SGLT2 transport proteins in the proximal convoluted tubule (PCT) in the kidney. Because these transporters account for nearly 90% of all filtered-glucose reabsorption in the body, they are excellent targets for blood-glucose control. SGLT2 inhibitors have been associated with HbA1c reductions of 0.5% to 1%, making them effective second-line treatment options for T2DM.6
Aside from their effectiveness in diabetes management, SGLT2 inhibitors have demonstrated additional benefits, including weight loss and management of macrovascular and microvascular complications secondary to T2DM.7-9 More specifically, SGLT2 inhibitors have demonstrated positive CV outcomes.
CV Effects of SGLT2 Inhibitors
SGLT2s, a family of transport proteins, exist in both the intestinal mucosa of the small intestine and the proximal tubule of the nephron. As noted above, SGLT2 inhibitors act on the SGLT transporters, which are located in the PCT.6 Promotion of glucose excretion causes plasma glucose levels to decrease, leading to HbA1c improvement. Additionally, owing to a caloric loss resulting from promoted glucose excretion, weight loss occurs in most patients with normal renal function. Glycosuria resulting from SGLT2 inhibitors could also mediate a uricosuric effect by way of the Glut-9 transporter; increased levels of uric acid are often associated with congestive HF.10
Other possible advantages for HF patients have been observed with SGLT2 inhibitor use. Natriuresis is expected to occur in patients receiving SGLT2 inhibitor therapy because SGLT2s in the PCT account for about 5% of the body’s sodium reabsorption.11 Natriuresis, combined with diuresis, is associated with decreases in plasma volume, blood pressure (BP), vascular stiffness, preload and afterload, and cardiac-wall stress. A lessening of these factors is beneficial for patients with HF. Additionally, by acting in the PCT, SGLT2 inhibitors increase the delivery of fluid and electrolytes to the macula densa, which then triggers tubuloglomerular feedback. This feedback mechanism may account for the estimated 30% to 40% reduction in albuminuria in patients taking these drugs, which could cause kidney disease and a higher risk of hospitalization for HF (HHF).12 By preserving the glomerular filtration rate (GFR) via this mechanism, the risk of HHF is decreased.
SGLT2 inhibitors also shift the balance of cardiac energy production in patients with T2DM. Typically, most cardiac energy (95%) derives from oxidative metabolism of free fatty acids and glucose, 60% to 70% from fatty-acid oxidation; in T2DM patients, the proportion is greatly increased.13 Because fatty-acid oxidation is much less efficient energetically, cardiac function is lessened in these patients. SGLT2 inhibitors improve cardiac function by promoting hepatic synthesis of ketones. Accordingly, beta-hydroxybutyrate can be taken up by both the heart and the kidney and is preferentially oxidized before fatty acids, resulting in a theoretical increase in cardiac function.14 SGLT2 inhibitors have also been shown to inhibit Na+/H+ exchanger-1 (NHE-1).15 Patients with T2DM and those with HF have higher NHE-1 activity, which subsequently can cause cardiomyocyte injury as well as decreased mitochondrial Ca2+, leading to decreased adenosine triphosphate (ATP) production in the heart. This can be a serious problem for HF patients because it reduces their cardiac output even further. SGLT2 inhibitors can inhibit NHE-1 even when they are not expressed in the heart. When NHE-1 is inhibited, mitochondrial Ca2+ is increased, resulting in increased ATP production in the heart.
Primary CV Outcomes
This section describes primary CV outcomes of SGLT2 inhibitors. See TABLE 1 for a summary of outcomes in the CANVAS (canagliflozin), EMPA-REG (empagliflozin), and DECLARE-TIMI (dapagliflozin) trials.
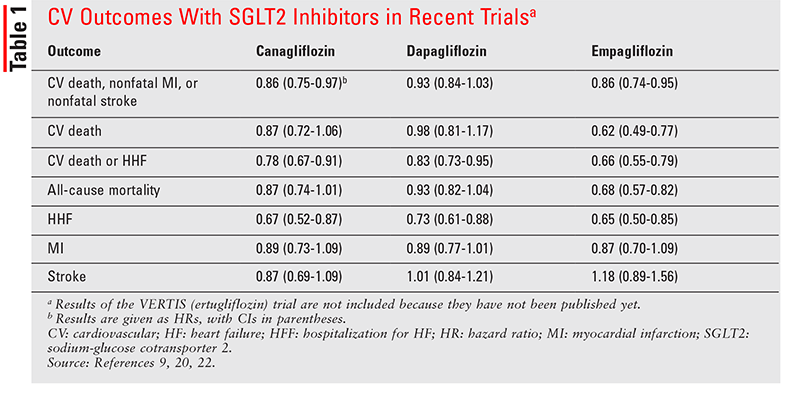
Empagliflozin: Empagliflozin was the first SGLT2 inhibitor evaluated for its CV impact (in EMPA-REG).9 The primary outcome was a composite of major adverse CV events (MACE): CV death, nonfatal myocardial infarction (MI), and nonfatal stroke. In 7,020 patients with T2DM and established ASCVD, empagliflozin not only was noninferior to placebo in meeting the primary endpoint, but also demonstrated superiority (10.5% vs. 12.1%, hazard ratio [HR] 0.86; 95% CI, 0.75-0.99; P <.001 for noninferiority, P = .04 for superiority). Compared with placebo, empagliflozin resulted in a significant reduction in HHF (9.4% vs. 14.5%, HR 0.65; 95% CI, 0.50-0.85; P = .002). Adverse events (AEs) such as hypoglycemia, urinary tract infections (UTIs), diabetic ketoacidosis, and acute renal failure were also tracked. As expected, UTIs were significantly more common in the empagliflozin group than in the placebo group.
The EMPA-REG OUTCOME investigators conducted further analyses of HF outcomes in patients with or without HF at baseline.16 Approximately 10% of the study population in both treatment arms had HF at baseline. HHF or CV death occurred significantly less among patients treated with empagliflozin compared with placebo (5.7% vs. 8.5%, HR 0.66; 95% CI, 0.55-0.79; P <.001). Additionally, empagliflozin improved HHF or death from HF (2.8% vs. 4.5%, HR 0.61; 95% CI, 0.47-0.79; P <.001). Patients receiving empagliflozin did not require loop diuretics as often as placebo patients did (HR 0.62; 95% CI, 0.53-0.73; P <.001). Compared with patients without HF at baseline, those with HF were two to six times more likely to exhibit an end outcome associated with HF; however, the risk reduction was similar in patients with and without HF at baseline. In both treatment arms, AEs occurred more frequently in patients with HF versus those without it.
The EMPERIAL-Reduced and EMPERIAL-Preserved trials are evaluating the effects of empagliflozin versus placebo on exercise ability in chronic HF with reduced ejection fraction (HFrEF) and preserved EF (HFpEF), respectively.17 The results of EMPERIAL-Reduced are currently being analyzed, and EMPERIAL-Preserved remains ongoing. The EMPEROR-Reduced and EMPEROR-Preserved trials are comparing the safety and efficacy of empagliflozin versus placebo in patients with HFrEF and HFpEF, respectively, who are already receiving guideline-directed medical therapy (GDMT).18,19 These four trials focus not on SGLT2 inhibitors’ antihyperglycemic impact on DM patients, but rather on their HF-associated mortality and morbidity impact in patients with existing HF.
Canagliflozin: The utility of canagliflozin in HF patients was examined in the CANVAS Program, which consisted of two double-blind, randomized, placebo-controlled trials: CANVAS and CANVAS-R.22 The program aimed to determine CV, renal, and safety outcomes of canagliflozin given its already-proven ability to reduce hyperglycemia, hypertension, and body weight. Subjects were men and women aged 30 years or older with T2DM and symptomatic ASCVD or those aged 50 years or older with risk factors for ASCVD. All subjects had to have an estimated GFR of at least 30 mL per minute to show normal renal function.
The primary outcome of CANVAS was a combination of death from CV causes, nonfatal MI, and nonfatal stroke.20 The secondary outcome was death from any cause, progression of albuminuria, and the combination of death from CV causes and HHF. At baseline, both groups had similar characteristics; additionally, HF subjects and non-HF subjects had similar characteristics.
In CANVAS, canagliflozin was associated with much lower risks of CV death or HHF compared with placebo (16.3 vs. 20.8 events per 1,000 patient-years, HR 0.78; 95% CI, 0.67-0.91; P <.002).20 Additionally, compared with placebo, canagliflozin had substantially lower risks of fatal or hospitalized HF (6.4 vs. 9.7 events per 1,000 patient-years, HR 0.70; 95% CI, 0.55-0.89; P <.003) and HHF alone (5.5 vs. 8.7 events per 1,000 patient-years, HR 0.67; 95% CI, 0.52-0.87; P <.002). The beneficial effect on the primary outcome was higher in patients with a prior history of HF compared with those without established HF at baseline.20 CANVAS evidently proved the efficacy of canagliflozin use to prevent HHF, especially in patients with established HF. These advantages became evident early in the follow-up period, suggesting that the mechanism of action benefiting HF is natriuresis, leading to decreased preload and afterload as well as lowering systemic BP and reducing arterial stiffness.21 Renal protection and cardiac-energy metabolism may also contribute to the reduction in HF risk.
Dapagliflozin: DECLARE-TIMI 58 studied the CV safety and efficacy of dapagliflozin in more than 17,000 patients with T2DM, approximately 40% of whom had established ASCVD and 60% of whom exhibited risk factors for ASCVD.22 About 10% of patients had a history of HF. The primary efficacy outcomes were MACE and a composite of CV death or HHF. The safety outcome was the composite of MACE. Dapagliflozin demonstrated noninferiority with regard to MACE (P <.001), but not a lower rate of MACE compared with placebo (8.8% vs. 9.4%, HR 0.93; 95% CI, 0.84-1.03; P = .17). However, there was a significant reduction of CV death or HHF (4.9% vs. 5.8%, HR 0.83; 95% CI, 0.73-0.95; P = .005). The reduction in the latter composite was driven largely by the 27% risk reduction in HHF. In subgroup analyses, patients with or without established ASCVD and those with or without HF history had similar reductions in CV death or HHF in the dapagliflozin arm versus the placebo arm. With respect to AEs, diabetic ketoacidosis and genital infections were more common in the dapagliflozin arm versus the placebo arm (0.3% vs. 0.1%, HR 2.18; 95% CI, 1.10-4.30; P = .02; and 0.9% vs. 0.1%, HR 8.36; 95% CI, 4.19-16.68; P <.001, respectively).22
A more recent study, DAPA-HF, evaluated dapagliflozin versus placebo in reducing the incidence of worsening HF or CV death in more than 4,700 patients with HFrEF receiving GDMT.23 About 42% of study patients had T2DM, and most patients (67%) had HF New York Heart Association functional class II. The 26% reduction in the primary outcome (16.3% vs. 21.2%, HR 0.74; 95% CI, 0.65-0.85; P <.001) was largely attributed to HHF.23 Secondary outcomes were also significant, including a lower occurrence of the composite of CV death or HHF (16.1% vs. 20.9%, HR 0.75; 95% CI, 0.65-0.85; P <.001) and fewer HF symptoms, according to higher scores on the Kansas City Cardiomyopathy Questionnaire (HR 1.18; 95% CI, 1.11-1.26; P <.001).23 In subgroup analyses of nondiabetic patients, dapagliflozin remained effective in achieving the primary outcome compared with placebo.
Ertugliflozin: VERTIS-CV, which was scheduled to be completed at the end of 2019, evaluated the safety and efficacy of ertugliflozin in patients with T2DM and established ASCVD.24 The primary study objective was the achievement of ertugliflozin’s noninferiority to placebo in MACE. One of the secondary objectives was the composite of CV death or HHF, which was similar to that in other SGLT2 inhibitors’ CV outcomes trials (CVOTs). Based on published results of baseline characteristics in 8,200 patients, approximately 23% of patients had HF; in about three-quarters of these patients with EF data, 80% exhibited HFpEF.24 Compared with CANVAS, EMPA-REG, and DECLARE, VERTIS-CV has the greatest number of enrolled patients with HF at baseline.
SGLT2 Inhibitors’ Role in HF Treatment
Current GDMT in patients with HF does not include the use of SGLT2 inhibitors. However, as stated earlier, patients with T2DM and ASCVD or HF may benefit from the addition of this medication class as an antihyperglycemic agent.
FIGURE 1 depicts the overall approach to selecting a SGLT2 inhibitor for a patient with T2DM. Results from the VERTIS (ertugliflozin) trial have not yet been published and therefore are not included here. All three SGLT2 inhibitors in FIGURE 1 confer a high risk of lower-limb amputation. A 5-year pharmacovigilance study found that both empagliflozin and canagliflozin had an increased risk of lower-limb amputation compared with placebo (proportional reporting ratio [PRR] 7.09; 95% CI, 5.25-9.57; and PRR 4.96, 95% CI, 2.89-8.50; respectively).25 However, in a post hoc analysis of the EMPA-REG OUTCOME trial, empagliflozin did not demonstrate an increased risk of lower-limb amputation.26 Similarly, CANVAS-R found no increased risk of lower-limb amputation with canagliflozin; the study was stopped early, however, and therefore the earlier findings of canagliflozin studies are still considered.
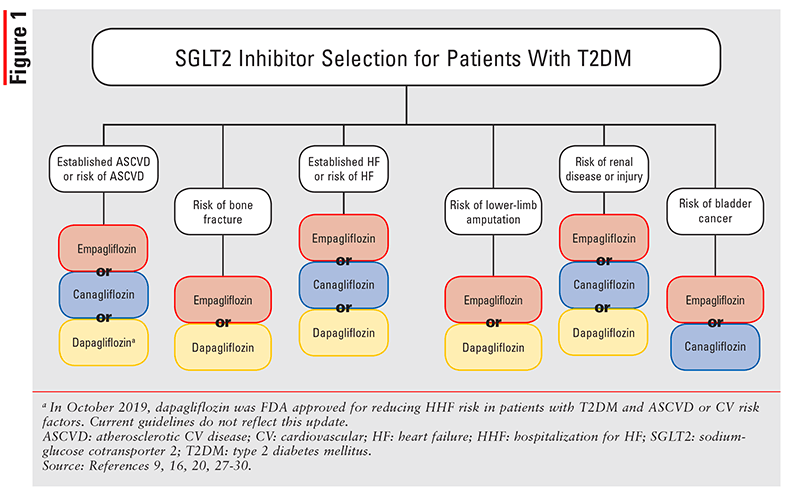
As CVOTs have demonstrated, SGLT2 inhibitors can prevent HHF in patients with or without HF at baseline, improve HF symptom scores, and reduce all-cause mortality; the rate of serious AEs is also very low. HF patients receiving GDMT take many medications to avoid hospitalization and prevent exacerbations. Although SGLT2 inhibitors may be a beneficial adjunct to GDMT, the pill burden and patient cost would be greater. Pharmacists are well positioned to discuss the advantages and disadvantages of these agents with prescribers and patients.
Conclusion
SGLT2 inhibitors are a novel class of antidiabetic agents that have demonstrated positive efficacy and safety outcomes in the setting of chronic HF. The FDA’s recent approval of dapagliflozin for reducing HHF may pave the way for other SGLT2 inhibitors to follow suit. The role of SGLT2 inhibitors in HF is promising and may inform future updates to guidelines for the management of HF.
REFERENCES
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S90-S102.
2. American College of Cardiology. ASCVD Risk Estimator Plus. Terms and concepts. http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/content/clinician-split-layout/terms_conditions. Accessed November 25, 2019.
3. Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294-e324.
4. Stone PH, Muller JE, Hartwell T, et al. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. J Am Coll Cardiol. 1989;14(1):49-57.
5. Nichols GA, Gullion CM, Koro CE, et al. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879-1884.
6. Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79.
7. Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79(3):219-230.
8. Davidson JA. SGLT2 inhibitors in patients with type 2 diabetes and renal disease: overview of current evidence. Postgrad Med. 2019;131(4):251-260.
9. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
10. Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643-1658.
11. Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752-772.
12. Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2012;5:313-327.
13. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75(1):33-59.
14. Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44(6):457-464.
15. Packer M, Anker SD, Butler J, et al. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2(9):1025-1029.
16. Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37(19):1526-1534.
17. Abraham WT, Ponikowski P, Brueckmann M, et al. Rationale and design of the EMPERIAL-Preserved and EMPERIAL-Reduced trials of empagliflozin in patients with chronic heart failure. Eur J Heart Fail. 2019;21(7):932-942.
18. Packer M, Butler J, Filippatos GS, et al. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail. 2019;21(10):1270-1278.
19. Anker SD, Butler J, Filippatos GS, et al. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail. 2019;21(10):1279-1287.
20. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
21. Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in type 2 diabetes mellitus. Circulation. 2018;138(5):458-468.
22. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357.
23. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008.
24. Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J. 2018;206:11-23.
25. Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: is this a class effect? Diabetes Obes Metab. 2018;20(6):1531-1534.
26. Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(1):e4-e5.
27. Rosenwasser RF, Sultan S, Sutton D, et al. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab Syndr Obes. 2013;6:453-467.
28. Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101(1):157-166.
29. Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:k4365.
30. FDA clears dapagliflozin to reduce heart failure hospitalizations. www.dicardiology.com/article/fda-clears-dapagliflozin-reduce-heart-failure-hospitalizations. Accessed November 27, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.





