US
Pharm. 2006;2:20-27.
Coronary heart disease (CHD)
is the leading cause of death in the United States, accounting for about one
third of deaths per year, regardless of gender.1 Due to increased
incidence and prevalence of the disease secondary to aging, 80% of the deaths
from CHD occur in people o
lder than 65 years.2 Based on the projected rapid growth of the
population older than 85 by 2030, a huge increase in the overall burden of CHD
is predicted.2
While care strategies for CHD
in elderly individuals are similar to those in younger patients, health care
providers should consider age-related differences, such as variation in
disease presentation, alterations in drug pharmacokinetics, more complex
disease management secondary to comorbid illness, and differences in patients'
preferences for care and health expectations, compared to those of younger
adults.3 Major complications of CHD include angina pectoris,
unstable angina, myocardial infarction (MI), and sudden cardiac death due to
arrhythmias.1 The plan of care for elderly patients with CHD must
be formulated with a patient-centered approach.3 This philosophy
should be balanced with a growing emphasis on maintaining or increasing
functional independence and quality of life for older individuals.2
Pathophysiology
While
patient-to-patient variability does not render CHD inevitable with advancing
age, certain cardiovascular changes are more commonly seen in older
individuals. Accumulated damage to the endothelium results in an increasing
number and severity of atherosclerotic plaques.3 These plaques, or
sub intimal deposits of lipids and connective tissue, reduce or obstruct
blood flow, causing CHD, cerebrovascular disease (e.g., stroke), and
peripheral vascular disease.4 The association between high
cholesterol levels and CHD is well established.4 This article will
focus on the special considerations for cholesterol management in older
persons.
Although cholesterol levels
increase with age, diet and genetic factors can also contribute to cholesterol
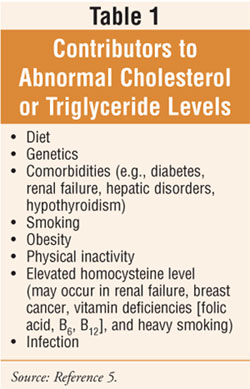
and triglyceride (TG) levels (table 1). A diet high in saturated fats, trans
fatty acids (i.e., artificially processed hydrogenated fats), and refined or
simple carbohydrates increases the total cholesterol level in most people.
5 Ingesting large quantities of alcohol and excess calories can also
raise TG levels. An individual's genetic factors influence the rate that fats
are manufactured, utilized, and eliminated in the body.
Dyslipidemia
The processing of
exogenous dietary fat in the gastrointestinal (GI) tract and the endogenous
synthesis and secretion of lipoproteins rich in TGs by the liver is known as
lipoprotein metabolism.2 The physiologic regulation of this
process provides an understanding of the pathogenesis of atherosclerosis and
the metabolic mechanisms by which therapies can reduce CHD risk.2
Disorders of lipoprotein
metabolism arise either from accelerated synthesis, decreased degradation of
the lipoproteins, or both.2 Dyslipoproteinemia may be primary
(e.g., single-gene mutation, polygenetic, multifactorial) or secondary to
other systemic disorders (e.g., diabetes mellitus, thyroid, renal, or liver
disease). It is unknown to what extent inactivity, secondary factors (e.g.,
abnormal body composition), and drugs (e.g., thiazide diuretics,
beta-adrenergic blockers, cyclosporine, glucocorticoids) may be related to
dyslipidemia.6,7
Lipoprotein particles contain
TG, cholesterol, cholesterol esters, phospholipids, and apolipoproteins. Five
classes of lipoprotein particles in the plasma are classified by their density
and include: chylomicrons (very-low-density lipoproteins, VLDLs),
intermediate-density lipoproteins (IDLs), low-density lipoproteins (LDLs), and
high-density lipoproteins (HDLs). Particles of LDL complexed to glycoprotein
apo(a), known as Lp(a), are also present in the plasma. In general,
total cholesterol, low-density lipo protein cholesterol (LDL-C), and TG
concentrations increase during the third to seventh or eighth decades of life
in both men and women, with a more pronounced increase in LDL-C
levels in women.2
According to the American Heart
Association (AHA), total cholesterol levels of 240 mg/dL or above in adults
are considered high, and levels from 200 to 239 mg/dL are considered
borderline-high.8 According to the National Health and Nutrition
Examination Survey III 1988–
1994, LDL-C levels of 130 mg/dL or above are associated with a higher risk of
CHD; HDL-C levels lower than 40 mg/dL are also associated with a higher risk
of CHD.8 TGs are considered elevated if they exceed 150 mg/dL after
an eight-hour fast.5 Secondary causes of hyperlipidemia, especially
diabetes mellitus (DM) and thyroid and renal disease, should be considered
when levels in multiple lipoprotein classes are abnormal in the older patient.
2
Therapeutic Interventions
Diet, weight
loss, and exercise:
Weight loss, aerobic exercise training, and diets reduced in saturated fat and
cholesterol (AHA Step 1 diet) are widely advocated for the initial treatment
of hyperlipidemia.2 The AHA diet provides the greatest absolute
improvements in hyperlipidemia; however, its universality in the primary
prevention of CHD in individuals older than 75 years and in some patients with
type 2 DM is questioned. While a strict lipid-lowering diet may be effective
in elderly patients, it may lead to or exacerbate malnutrition. In addition,
unappetizing low-fat diets can precipitate anorexia and lead to nutritional
deficiencies.4 Complications of protein energy malnutrition (e.g.,
pressure sores, anemia, decreased muscle strength, infections) must be weighed
against those of moderate hypercholesteremia on a patient-by-patient basis.
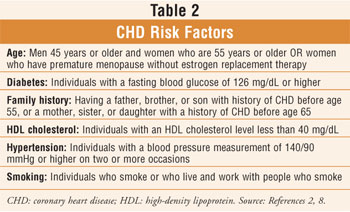
4 The positive effects of the AHA diet and aerobic exercise on other CHD
risk factors (table 2) and quality of life have been enhanced by weight loss
and should be considered, if appropriate, as part of the overall treatment
plan.2
Studies have shown fewer
atherogenic profiles associated with higher levels of physical activity in
young and middle-aged individuals.6 Although physical activity has
an apparent lack of effect on total cholesterol and LDL-C levels in many
studies, trained subjects consistently show reduced plasma TG and VLDL
concentrations, as well as more elevated HDL-C, HDL2-C, and Lp(a-1) levels, as
compared to sedentary controls.6 Many studies suggest, although not
all agree, that weight loss (or fat loss) in general is necessary to increase
HDL-C levels with exercise training.6 Vigorous exercise, with or
without hormone replacement therapy (HRT), has been examined in postmenopausal
women. Results indicate indepen dent effects of exercise and HRT, and
exercise alone mitigated the elevation of TG associated with HRT.6
Weight loss and a reduced risk
of developing hypertension may be achieved through aerobic exercise at least
30 minutes three times per week.9 Stress management techniques such
as yoga may help control high blood pressure (BP), while other relaxation
techniques (e.g., music therapy combined with deep breathing) have
demonstrated a reduction in BP in hypertensive patients.9 Clinical
trials with long-term follow-up are necessary to determine if these lifestyle
interventions will decrease the risk of CHD and overall morbidity and
mortality in patients older than 65 years.
Pharmacologic treatment:
Pharmacologic intervention can be considered for individuals who continue to
require treatment for hypercholesteremia despite adequate dietary therapy,
regular physical activity, and weight loss. Drug treatment strategies for
hyperlipidemia are directed at key regulatory sites of lipoprotein metabolism.
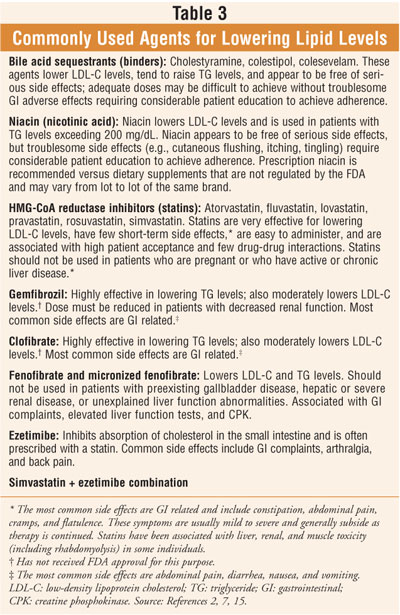
2 Several classes of agents are marketed for lowering serum cholesterol (
table 3). These agents appear to be as efficacious in the elderly as in
middle-aged people, and numerous clinical trials of lipid-lowering therapy
found substantial reductions in cardiac morbidity and mortality in the "young"
elderly (ages 65 to 75 years).2,3 Many researchers believe that in
the "older" elderly (persons older than 75 years), pharmacologic treatment
should generally be reserved for patients with overt CHD or atherosclerotic
vascular disease, or for patients with multiple CHD risk factors in whom the
benefits of treatment have been balanced against the potential risks and costs.
2 In a vigorous "older" elderly individual, treatment for
primary prevention may be reasonable as well.2 LDL-C levels
associated with the goals and considerations of drug therapy are listed in
table 4.10
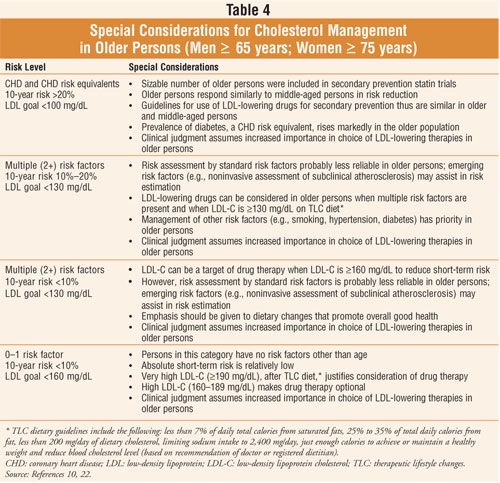
It is important to note that
assessments using Framingham risk prediction scores (a method of identifying
individuals at risk for CHD) may be less reliable in seniors.10 A
partial solution is the measurement of subclinical atherosclerosis by
noninvasive techniques.10 If an older person is found to have
advanced coronary or systemic atherosclerosis, LDL-lowering therapy can be
intensified even in the absence of clinical coronary symptoms. The relative
risk of hyperlipidemia for CHD is lower in seniors due to competing causes of
mortality and cumulative effects of comorbid illness. However, due to a high
absolute risk in the elderly and the high attributable risk for CHD events,
older patients with CHD and multiple risk factors are particularly expected to
benefit from pharmacologic intervention.2 Treatment such as HMG-CoA
reductase inhibitor (statin) therapy will prevent a greater number of events
in older patients than in their younger counterparts due to a high absolute
risk and a high attributable risk for CHD in seniors.2 A recent
literature search found that for women with known CHD, pharmacologic treatment
of hyperlipidemia is effective in reducing CHD events, CHD mortality, nonfatal
MI, and revascularization but does not affect total mortality.11
The use of pharmacologic
intervention for hyperlipidemia in elderly patients should be carefully
balanced with factors against such treatment, including: (1) a potential lag
time (two years) between initiation of therapy and reduction of morbidity and
mortality from CHD; (2) possible greater drug side effects; (3) cost of
treatment for el derly persons with limited insurance and a fixed income; (4)
the presence of comorbid illnesses limiting life span or quality of life; (5)
polypharmacy and the risk of side effects; (6) lack of evidence that primary
or secondary prevention decreases CHD morbidity and mortality in individuals
older than 75 years.2,4
Metabolic Syndrome
While alterations
in lipoprotein metabolism are most apparent in patients with diabetes, they
are often present to a lesser degree in patients with metabolic syndrome
(i.e., visceral [truncal] obesity, hyperinsulinemia, elevated blood glucose
levels, low HDL-C, elevated TG, hypertension, and prothrombic state
contributing to an increased CHD risk).2,12 These individuals do
not yet manifest a degree of hyperglycemia that supports a diagnosis of
diabetes. As mentioned above, secondary causes for hyperlipidemia (especially
DM, thyroid disease, and renal disease) should be considered when multiple
lipoprotein classes are abnormal in an older patient.2 Incidents of
atypical antipsychotic (AAP)-induced hypertriglyceridemia have been reported,
although the biochemical causes are still unclear.13 One study
summarized 14 cases of severe hypertriglyceridemia (TG >600 mg/dL) with a
subset of those patients who also developed new-onset DM.13 The
identified cases occurred within three to eight months of treatment with an
AAP medication, and the documented weight gain (8.5 to 12.3 lb) did not
correlate with the severity of hypertriglyceridemia.
Monitoring and Patient
Education
In general,
lipid-lowering agents are safe in seniors, but routine monitoring is
recommended. With the initiation of statin therapy, testing for liver, renal,
and muscle toxicity should be performed monthly for three months (including
baseline), then every six months and following
a dose increase. Quarterly monitoring is recommended when combination therapy
is used.2,3,7 Assessing the interactions between hypolipidemic
agents and other prescribed and over-the-counter medications and vitamins is
essential. The potential benefits of statins and niacin may be altered with
concomitant supplemental antioxidant vitamin therapy.2 The most
common cause of drug-induced rhabdomyolysis is direct myotoxicity from statins.
14 This condition leads to intratubular precipitation of myoglobin and,
if severe, results in acute renal failure. The risk of rhabdomyolysis is
increased when statins are concomitantly administered with gemfibrozil,
niacin, or CYP3A4 inhibitors (e.g., erythromycin, itraconazole, cyclo
sporine).14 Baseline and follow-up creatine phosphokinase (CPK)
measurements every six months have been used to identify patients who develop
subclinical rhabdomyolysis while receiving lipid-lowering therapy.7
In general, patients receiving
statins should be instructed to report excessive or acute muscle cramping or
weakness, unresolved diarrhea, changes in mood or memory, yellowing of skin or
eyes, easy bruising or bleeding, and unusual fatigue to their health care pro
vider.15 Patients should be instructed to take niacin with food to
avoid GI upset and should be advised that flushing and sensation of warmth,
especially of the face and upper body, may occur; headache and itching or
tingling have also been reported.15
Patients treated with AAPs
should be considered at high risk of developing DM and should be routinely
screened for DM and other metabolic abnormalities, including hyperlipidemia.
16-18 Close monitoring of weight, fasting plasma glucose, and serum
lipid levels should be considered during extended treatment with AAPs.
13,18,19 Many clinicians advocate intervention at the first sign of DM
onset or im paired glucose tolerance.20 Glucose metabolism
impairment is a major area of concern requiring attention by the psychiatric
team and primary care clinicians.21 Pharmacists are well positioned
to take a proactive role in identifying patients at risk and to participate in
vigilant monitoring and patient/caregiver education.
Hypocholesteremia
Hypocholesteremia,
usually defined as total cholesterol levels less than 160 mg/dL, has been
studied in older individuals and associated with increased death from
intracranial hemorrhage, lymphatic and hematopoietic cancers, chronic
obstructive pulmonary disease (particularly in smokers), and cirrhosis.2
Older adults were also at an increased risk for adverse events. Low
cholesterol levels may indicate an acute illness associated with the release
of cytokines.4 When total cholesterol levels fall below 160 mg/dL
in nursing home patients, mortality is predicted, presumably because such low
levels reflect malnutrition.4 Biological mechanisms that may
explain low total cholesterol as a cause of death include alterations in cell
membrane structure and function, abnormalities in steroid hormone metabolism,
and fat and vitamin deficiencies.2
Conclusion
Despite advances in
the treatment and prevention of CHD, the disease continues to be a major
health problem and the most common causeof death in old age. The prevalence
and mortality rates of CHD increase with age, and the association between high
cholesterol levels and CHD is well established. There is much interest and
need for research to further explore the efficacy and safety of treatment and
prevention strategies for CHD in older adults. While substantial evidence
supports the use of lifestyle and pharmacologic interventions in treating
hyperlipidemia in the elderly, the National Cholesterol Education Program
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol
in Adults acknowledges that clinical judgment plays a critical role in
developing the appropriate management strategy for these individuals.10
REFERENCES
1. Beers MH, Berkow
R, eds. The Merck Manual of Diagnosis and Therapy. 17th ed. Whitehouse
Station, NJ: Merck & Co.; 1999:206-211.
2. Katzel LI, Goldberg
AP. Dyslipoproteinemia. In: Hazzard WR, Blass JP, Halter JB, et al.
Principles of Geriatric Medicine and Gerontology. 5th ed. New York:
McGraw-Hill Inc.; 2003:875-891.
3. Peterson E,
Bensimhon DR. Coronary heart disease. In: Hazzard WR, Blass JP, Halter JB, et
al. Principles of Geriatric Medicine and Gerontology. 5th ed. New York:
McGraw-Hill, Inc.; 2003:433-444.
4. Beers MH, Berkow R,
eds. The Merck Manual of Geriatrics. 3rd ed. Whitehouse Station, NJ: Merck &
Co; 2000:595-596, 600-601, 606-624.
5. Beers MH, Jones TV,
Berkwits M, et al, eds. The Merck Manual of Health & Aging.
Whitehouse Station, NJ: Merck Research Laboratories; 2004:551-560.
6. Schwartz RS, Kohrt
WM. Exercise in elderly people: physiologic and functional effects. In:
Hazzard WR, Blass JP, Halter JB, et al. Principles of Geriatric Medicine
and Gerontology. 5th ed. New York: McGraw-Hill, Inc.; 2003:931-941.
7. Johnson HJ,
Heim-Duthoy KL. Renal transplantation. In: DiPiro JT, Talbert RL, Yee GC, et
al, eds. Pharmacotherapy: A Pathophysiologic Approach. 5th ed. New
York: McGraw-Hill; 2002:861.
8. American Heart
Association. Cholesterol-Lowering Drugs. Available at:
www.americanheart.org/presenter. jhtml?identifier=557. Accessed December 21,
2005.
9. Wertkin AD, Cizza G,
Blackman MR. Complimentary and alternative medicine in aging. In: Hazzard WR,
Blass JP, Halter JB, et al. Principles of Geriatric Medicine and Gerontology
. 5th ed. New York: McGraw-Hill, Inc.; 2003:242.
10. National
Cholesterol Education Program Expert Panel on Detection, Evaluation, and
Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).
National Cholesterol Education Program Adult Treatment Panel III Report. 2001.
Available at: www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf. Accessed
January 10, 2006.
11. Walsh JM, Pignone
M. Drug treatment of hyperlipidemia in women. JAMA. 2004;291:2243-2252.
12. Hazzard WR, Chang
MY, Chait A. Aging and atherosclerosis. In: Hazzard WR, Blass JP, Halter JB,
et al. Principles of Geriatric Medicine and Gerontology. 5th ed. New
York: McGraw-Hill, Inc.; 2003:423-431.
13. Meyer JM. Novel
antipsychotics and severe hyperlipidemia. J Clin Psychopharmacol.
2001;21:369-374.
14. Nolin TD, Abraham
PA, Matzke GR. Drug-induced renal disease. In: DiPiro JT, Talbert RL, Yee GC,
eds, et al. Pharmacotherapy: A Pathophysiologic Approach. 5th ed. New
York: McGraw-Hill; 2002:900.
15. Semla TP, Beizer
JL, Higbee MD. Geriatric Dosage Handbook. 10th ed. Cleveland:
Lexi-Comp, Inc.; 2005:112-113, 281-283, 489-491, 887-889.
16. Henderson DC.
Atypical antipsychotic-induced diabetes mellitus: how strong is the evidence?
CNS Drugs. 2002;16:77-89.
17. Ananth J, Venkatesh
R, Burgoyne K, Gunatilake S. Atypical antipsychotic drug use and diabetes.
Psychother Psychosom. 2002;71:244-254.
18. Wirshing DA, Pierre
JM, Eyeler J, et al. Risperidone-associated new-onset diabetes. Biol
Psychiatry. 2001;50:148-149.
19. Meyer JM. A
retrospective comparison of weight, lipid, and glucose changes between
risperidone- and olanzapine-treated inpatients: metabolic outcomes after 1
year. J Clin Psychiatry. 2002;63:425-433.
20. Sernyak MJ,
Gulanski B, Leslie DL, Rosenheck R. Undiagnosed hyperglycemia in
clozapine-treated patients with schizophrenia. J Clin Psychiatry.
2003;64:605-608.
21. Henderson DC.
Clinical experience with insulin resistance, diabetic ketoacidosis, and type 2
diabetes mellitus in patients treated with atypical antipsychotic agents. J
Clin Psychiatry. 2001;62(suppl 27):10-14.
22. National Heart,
Lung, and Blood Institute. National Institutes of Health. Therapeutic
Lifestyle Changes Diet. Available at: www.nhlbi.nih.gov/cgi-bin/chd/
step2intro.cgi. Accessed January 16, 2006.
To comment on this article,
contact
editor@uspharmacist.com.






