US Pharm. 2020;45(4):HS-2-HS-HS-6.
ABSTRACT: Pain is a complex medical syndrome commonly encountered in the ICU. Inadequate treatment of acute pain can lead to a prolonged need for sedation, increased days on the ventilator, and longer hospital stays. The opioid epidemic has led to increased recognition of the need for multimodal pain regimens involving adjunctive nonopioid drugs. Subanesthetic ketamine dosing has been studied for its analgesic properties and has become increasing popular as an opioid-sparing agent, especially in the ICU setting
Providing adequate pain therapy in the intensive-care setting has always been challenging, but in this era of opioid misuse and abuse, the issue has become even more complicated. Pain occurs when tissue damage from illness, injury, surgical procedures, or other noxious stimuli causes a release of inflammatory mediators such as bradykinin, histamine, prostaglandins, and substance P. These mediators can lead to hyperalgesia (increased sensitivity to pain) and allodynia (pain response to normally non-painful stimulation) by binding to and activating post-synaptic nerve terminals such as N-methyl-d-aspartic acid (NMDA) receptors.1
Pain is a common complaint of patients admitted to medical, surgical, and trauma ICUs; despite this, pain continues to be undertreated and underrecognized. In the ICU setting, inadequate pain treatment can lead to numerous detrimental effects. Some examples, such as impaired wound healing, infections, prolonged mechanical ventilation, physiological stressors, and psychological stressors, can lead to more extended hospital stays.2,3 It has also been reported that up to 44% of patients hospitalized in intensive-care settings experience chronic pain following hospital discharge.2,4 Therefore, appropriate pain treatment to reduce adverse outcomes related to pain, and potentially prevent progression to chronic pain, is essential.
In 2017, the U. S. Department of Health and Human Services (HHS) declared an opioid crisis. Although this epidemic is predominantly identified through outpatient overprescribing, opioid initiation during hospitalization is a recognized catalyst. The 60% increase in the cost of ICU admissions related to opioid overdoses between 2009 and 2015 highlights the impact this societal crisis has on acute-care settings.5 Even with this recognition, the 2018 PADIS (Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU) guidelines found that opioids continue to be the mainstay of pain control in the ICU setting.6 Because of the unfavorable side-effect profile of opioids (i.e., respiratory depression, delirium, ileus, nausea and vomiting, hypotension, tolerance), PADIS suggests using the lowest dose possible, which may subjectively lead to underdosing and inadequate analgesia.6 Ultimately, an individualized, multimodal approach with adjunctive nonopioid analgesics (e.g., ketamine, nonsteroidal anti-inflammatory drugs [NSAIDs], gabapentin) may be useful to control pain and help overcome the negative effects associated with higher doses of single agents.6 Subanesthetic ketamine dosing has been studied for its analgesic properties and has become increasingly popular as an opioid-sparing agent, especially in the ICU setting.
Pharmacology and Pharmacokinetics
Ketamine, a phencyclidine derivative possessing profound anesthetic, analgesic, and amnestic properties, was developed in 1962.7-9 It is approved by the FDA as an anesthetic induction agent for use before other general anesthetics or as monotherapy anesthesia for surgical procedures that do not require skeletal muscle relaxation.7 The FDA package insert for ketamine recommends an anesthetic induction dose that ranges from 1 mg/kg to 4.5 mg/kg IV, noting the average is 2 mg/kg.Ketamine’s primary mechanism of action is noncompetitive reversible NMDA receptor inhibition.10 Inhibiting NMDA receptors leads to a reduction in glutamate, which is the primary excitatory neurotransmitter in the central nervous system. This reduction in glutamate causes dissociation of the cerebral cortex and limbic system from sensory stimuli, which is responsible for its anesthetic, analgesic, and amnestic properties.11 It is also thought that ketamine works on other pain receptors such as the mu-opioid, gamma-aminobutyric acid, and muscarinic receptors. Data regarding ketamine use and its analgesic effects through non-NMDA receptors are limited.3,10,12
Only parenteral formulations of ketamine are commercially available in the U.S.; however, oral preparations can be compounded. When administered via IV route, ketamine crosses the blood-brain barrier rapidly, has a quick onset (~30 seconds), and a has short duration of action (~5 to 30 minutes).9,11,13 Ketamine is water- and lipid-soluble, and so it readily distributes to all highly perfused tissues after administration. It is hepatically metabolized to its major active metabolite, norketamine, which provides approximately one-third the analgesia of ketamine and is excreted primarily in the urine.2,9 IM ketamine is 93% bioavailable, but because of extensive first-pass metabolism, the oral bioavailability of ketamine is only 17% to 25%.9,13
What Is Subanesthetic Ketamine?
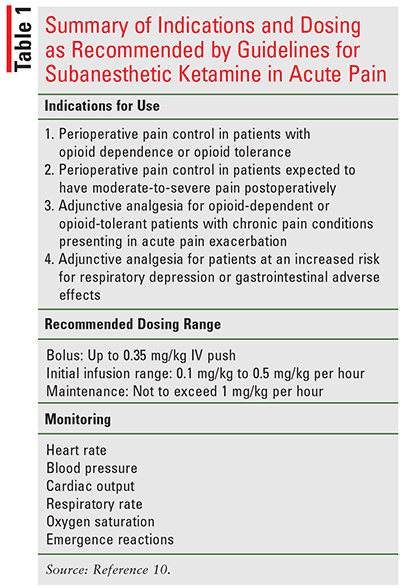
Despite the growing popularity of subanesthetic ketamine (<0.5 mg/kg) for chronic pain and severe depression, its use for acute pain is still relatively uncommon.13 The analgesic effects of ketamine occur at plasma concentrations of 100 ng/mL to 200 ng/mL compared with plasma concentrations of 9,000 ng/mL to 25,000 ng/mL that are required to induce and maintain surgical anesthesia.8,10 Although analgesic dosing for ketamine is not clearly defined in the literature, the PADIS guidelines and the 2018 American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists (ASRA/AAPM/ ASA) consensus guidelines for IV ketamine infusions recommend a bolus dose of ²0.35 mg/kg IV push. This may be followed by an infusion of 0.1 mg/kg to 0.2 mg/kg (not to exceed 1 mg/kg) per hour.6,8,10
Who May Benefit From Subanesthetic Ketamine?
A 2010 randomized, controlled trial by Loftus and colleagues assessed pain controlled and the opioid-sparing effect of using IV ketamine as compared with placebo in 102 opioid-dependent or opioid-tolerant patients after major back surgery.14 The total opioid dose after 24 and 48 hours and at a 6-week follow-up visit was significantly reduced in the ketamine group in comparison with the placebo group. However, other studies assessing postoperative pain control with ketamine in a similar population demonstrated little to no benefit.15-17 The lack of benefit may reflect the low number of patients included in the trials. Despite these negative studies, guidelines suggest that ketamine may be beneficial in this population even if it only provides minimal pain control.10
A 2011 systematic review by Laskowski and colleagues analyzed 47 double-blind, randomized trials that compared IV ketamine to placebo for adjunctive postoperative pain management.18 They concluded that across all studies, patients who received ketamine showed a significant reduction in total postoperative opioid use. Additionally, 78% of the patients who received ketamine had less overall postoperative pain compared with those who received placebo. They also noted a significant increase in time to first postoperative analgesic use in the ketamine group. Lastly, the greatest analgesic benefits from adjunctive ketamine use were observed in patients who underwent the more painful surgeries (thoracic, upper abdominal, major orthopedic) as compared with surgeries in which mild pain was expected.
In contrast to the negative respiratory effects associated with opioids (respiratory depression, rigid chest), ketamine appears to have beneficial effects on respiration by increasing endogenous catecholamines. Catecholamines bind to beta receptors in the lungs leading to bronchodilation and improved airway resistance.3,19 With common clinical doses, ketamine has minimal effects on airway reflexes, and in comparison to opioids, it is less likely to produce respiratory depression.8,19,20 Unlike NSAIDs, ketamine does not cause gastrointestinal bleeding. Although ketamine affects the mu-opioid receptors, it has not been linked to reduced gastric mobility, as seen with opioids.20 These properties make it an appealing alternative analgesic agent, especially for critically ill patients who are at an increased risk for respiratory depression and gastrointestinal bleeding.10
Other patient populations that may benefit from subanesthetic ketamine for pain management include those with chronic pain conditions (e.g., chronic pain syndromes, sickle cell disease), those with serious nonoperative trauma (e.g., rib fractures), or those who are dependent on opioids but present with acute pain exacerbations.8,10 To date, no randomized, controlled studies using ketamine for acute pain in these populations have been reported. There are case reports in patients with sickle cell crisis that showed improvements in acute pain after receiving ketamine.10,21-23
The PADIS guidelines and the ASRA/AAPM/ASA guidelines on the use of IV ketamine for acute pain recommend that ketamine be used in patients undergoing surgeries in which severe postoperative pain is expected or in opioid-tolerant or -dependent patients who present for surgery (TABLE 1).6,10 They also suggest ketamine be considered for opioid-dependent or -tolerant nonsurgical patients with chronic pain conditions who have acute pain exacerbations or in those patients at increased risk of respiratory depression or ileus.6,9,10
Safety of Subanesthetic Ketamine
Despite its advantages, ketamine does cause adverse effects on the central nervous and cardiovascular systems that may limit use in certain patients. It has dissociative (i.e., detachment from the subject’s immediate environment) and psychomimetic properties that have been well characterized when ketamine is used for procedural sedation.20,24 It is hypothesized that these properties are due to ketamine’s effect on the limbic, hippocampal, and thalamocortical systems, which lead to alterations in the perception of visual and auditory stimuli.8,12 Up to 30% of patients undergoing procedural sedation have “emergence reactions” characterized by hallucinations and delirium after ketamine use.12,20 Although subanesthetic doses (<0.5 mg/kg) have been reported to cause dissociation, these effects are more likely in patients who receive anesthetic dosages (>2 mg/kg), in those who have a history of psychiatric issues, and in those who have received opioids or other sedatives.2,12 Because of these psychomimetic reactions, ketamine has been classified by the Drug Enforcement Administration as a Schedule III controlled agent, as it has the potential to be addictive, and ketamine abuse has been widely reported.
Ketamine causes a dose-dependent release of endogenous catecholamines mediated through the sympathetic nervous system.25 Higher doses of ketamine have been reported to cause transient tachycardia and hypertension, which is a concern in patients with preexisting cardiovascular disease.2 While the effects on heart rate and blood pressure may be advantageous for patients who are hypotensive, this is not a driving factor in ketamine use. These sympathomimetic effects have not been elucidated in patients with prolonged illnesses who are catecholamine-depleted, and they theoretically lead to myocardial depression.2,3,20 Ketamine also has negative inotropic properties and should be avoided in patients with cardiovascular conditions such as heart failure or those at risk for myocardial infarction.2,20
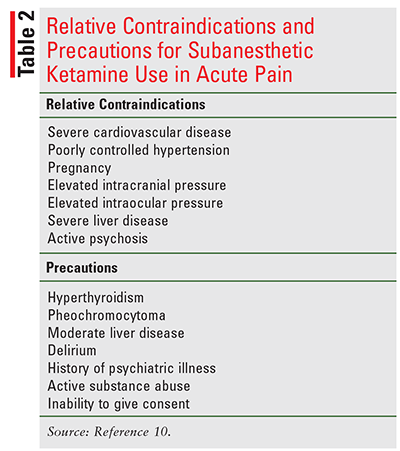
The majority of studies assessing subanesthetic ketamine use have enrolled relatively healthy patients or have excluded patients with contraindications to anesthetic doses of ketamine.10 The contraindications listed for subanesthetic ketamine have been extrapolated from anesthetic doses and are generally considered “relative.”8,10 A complete list of relative contraindications and other precautions for the use of subanesthetic ketamine in acute pain are listed in TABLE 2.
Pharmacist Considerations
In light of the opioid epidemic and the push to decrease opioid use, the desire to identify alternatives to opioids has sparked increased use of subanesthetic ketamine for acute pain in the ICU setting. Ketamine has shown efficacy in perioperative patients and those with acute pain. It is imperative for pharmacists, as well as other healthcare providers, to recognize the benefits and challenges associated with ketamine use.
REFERENCES
1. Argoff C. Mechanisms of pain transmission and pharmacologic management. Curr Med Res Opin. 2011;27(10):2019-2031.
2. Wampole CR, Smith KE. Beyond opioids for pain management in adult critically ill patients. J Pharm Pract. 2019;32(3):256-270.
3. Ehieli E, Yalamuri S, Brudney CS, Pyati S. Analgesia in the surgical intensive care unit. Postgrad Med J. 2017;93(1095):38-45.
4. Battle CE, Lovett S, Hutchings H. Chronic pain in survivors of critical illness: a retrospective analysis of incidence and risk factors. Crit Care. 2013;17(3):R101.
5. Stevens JP, Wall MJ, Novack L, et al. The critical care crisis of opioid overdoses in the United States. Ann Am Thorac Soc. 2017;14(12):1803-1809.
6. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
7. FDA. Ketalar (ketamine hydrochloride) injection. www.accessdata.fda.gov/drugsatfda_docs/ label/2017/016812s043lbl.pdf. Accessed October 3, 2017.
8. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
9. Mazzeffi M, Johnson K, Paciullo C. Ketamine in adult cardiac surgery and the cardiac surgery intensive care unit: an evidence-based clinical review. Ann Card Anaesth. 2015;18(2):202-209.
10. Schwenk ES, Viscusi ER, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):456-466.
11. Walters MK, Farhat J, Bischoff J, et al. Ketamine as an analgesic adjuvant in adult trauma intensive care unit patients with rib fracture. Ann Pharmacother. 2018;52(9):849-854.
12. Pruskowski KA, Harbourt K, Pajoumand M, et al. Impact of ketamine use on adjunctive analgesic and sedative medications in critically ill trauma patients. Pharmacotherapy. 2017;37(12):1537-1544.
13. Davis WD, Davis KA, Hooper K. The use of ketamine for the management of acute pain in the emergency department. Adv Emerg Nurs J. 2019;41(2):111-121.
14. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
15. Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg. 2004;99(2):482-495.
16. Urban MK, Ya Deau JT, Wukovits B, Lipnitsky JY. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: a prospective randomized trial. HSS J. 2008;4(1):62-65.
17. Vaid P, Green T, Shinkaruk K, King-Shier K. Low-dose ketamine infusions for highly opioid-tolerant adults following spinal surgery: a retrospective before-and-after study. Pain Manag Nurs. 2016;17(2):150-158.
18. Laskowski K, Stirling A, Mckay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58(10):911-923.
19. Patanwala AE, Martin JR, Erstad BL. Ketamine for analgosedation in the intensive care unit: a systematic review. J Intensive Care Med. 2017;32(6):387-395.
20. Erstad BL, Patanwala AE. Ketamine for analgosedation in critically ill patients. J Crit Care. 2016;35:145-149.
21. Uprety D, Baber A, Foy M. Ketamine infusion for sickle cell pain crisis refractory to opioids: a case report and review of literature. Ann Hematol. 2014;93(5):769-771.
22. Meals CG, Mullican BD, Shaffer CM, et al. Ketamine infusion for sickle cell crisis pain in an adult. J Pain Symptom Manage. 2011;42(3):e7-e9.
23. Tawfic QA, Faris AS, Kausalya R. The role of a low-dose ketamine-midazolam regimen in the management of severe painful crisis in patients with sickle cell disease. J Pain Symptom Manage. 2014;47(2):334-340.
24. Corssen G, Miyasaka M, Domino EF. Changing concepts in pain control during surgery: dissociative anesthesia with CI-581. A progress report. Anesth Analg. 1968;47(6):746-759.
25. Tobias JD, Leder M. Procedural sedation: a review of sedative agents, monitoring, and management of complications. Saudi J Anaesth. 2011;5(4):395-410.
To comment on this article, contact rdavidson@uspharmacist.com.