US Pharm. 2021;47(3):HS-11-HS-16.
ABSTRACT: The novel severe acute respiratory syndrome coronavirus 2, first discovered in December 2019, has presented with many challenges, impacting individuals globally with many requiring ICU admission and the need for invasive mechanical ventilation secondary to severe acute respiratory distress syndrome. To facilitate appropriate ventilator management, pain and sedation strategies as well as neuromuscular blocking agents are often utilized.
In December 2019, the first case of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred in Wuhan, China, the capital of Hubei Province.1 According to the CDC, more than 13.8 million people had tested positive in the United States alone as of December 2020.2 Although there is wide variability globally, more than 70% of those diagnosed with COVID-19 who become critically ill have required intubation and support with invasive mechanical ventilation.3,4
Achieving appropriate analgesia and sedation goals in mechanically intubated critically ill patients with COVID-19 has been challenging due to drug shortages as well as aggressive ventilator settings that may require deep sedation or paralysis to mitigate ventilator asynchronies. The clinical practice guidelines from the Society of Critical Care Medicine (SCCM) recommend a strategy of light sedation over deep sedation as well as utilization of nonbenzodiazepines (i.e., propofol or dexmedetomidine) in most critically ill patients to decrease time to extubation, tracheostomy rate, and ICU length of stay (LOS).5 While this strategy is normally followed appropriately, analgesia and sedation in patients with acute respiratory distress syndrome (ARDS) can be challenging as patient respiratory drive may potentiate ventilator asynchronies, increasing the risk of inappropriate oxygenation and ventilation. Furthermore, patients with ARDS are frequently proned to improve gas exchange and lung compliance. When in the prone position, they may experience pain, and the risk of endotracheal tube misplacement during proning maneuvers may lead to poor outcomes. Thus, those with ARDS due to COVID-19 are frequently deeply sedated.
This change in practice has led to nationwide drug shortages and the potential for increased rate of delirium and ICU-acquired weakness (ICUAW). This review aims at discussing the challenges of analgesia and sedation in patients with COVID-19 who require mechanical ventilation.
Analgesia and Sedation Strategies
Critically ill patients experience pain due to myriad causes, including underlying disease states, respiratory disease, invasive procedures, and endotracheal tube positioning and suctioning. Opioids remain the gold standard for the treatment of pain in critically ill patients, including those with COVID-19. The most commonly used IV opioids in the ICU setting include fentanyl, hydromorphone, and morphine (TABLE 1). In relation to COVID, it is important to be cognizant of several adverse effects with regards to opioid options. At high doses and rapid infusion, fentanyl administration has been associated with chest wall rigidity, which can decrease compliance and lead to inappropriate ventilation—a potentially devastating complication in the critically ill patient with COVID-19.6 Additionally, fentanyl undergoes CYP3A4 metabolism, which could lead to accumulation in patients with hepatic dysfunction.
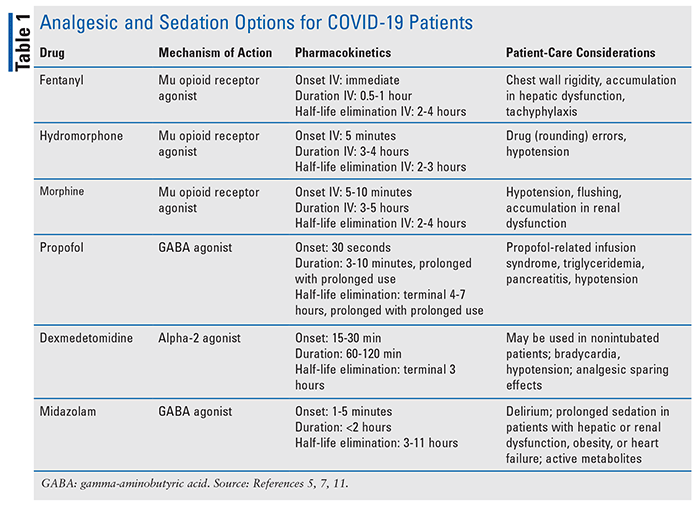
One advantage of fentanyl might be the ability to administer via the transdermal route. However, the absorption would be expected to be variable and delayed in ICU patients, considering changes in volume of distribution and absorption. Hydromorphone may be preferred in patients with renal or hepatic failure because inactive metabolites are produced after glucuronidation. Of note, these inactive metabolites have the potential to cause neurotoxicity in kidney dysfunction, but this is not noted in clinical practice at the doses prescribed. Morphine is most commonly utilized in patients at end of life due to adverse effects, including the potential of histamine release leading to hypotension. Morphine also has active metabolites that can accumulate in the setting of renal failure, leading to neurotoxicity.
Analgesia Amid Drug Shortages
Drug shortages during the pandemic have led clinicians to investigate other strategies for analgesia. Utilization of shorter acting opioids, such as remifentanil, sufentanil, and alfentanil, has been considered.7 Remifentanil has been compared to fentanyl in a randomized, controlled trial assessing analgosedation in critically ill, intubated patients. Remifentanil was found to be similar in efficacy but with even less dosing variability among patients relative to fentanyl. It was associated with increased pain periextubation, likely secondary to a short duration of action given its rapid metabolism by plasma esterases.7-9 Sufentanil and alfentanil are ultra-short–acting opioids that may also be considered for use in this setting. Enteral opioids, such as oxycodone, may also be an option to minimize the usage of IV opioids in the setting of drug shortages.10
Nonopioid analgesic options have also been explored. This includes agents such as acetaminophen, gabapentin, cyclobenzaprine, and pregabalin, which are commonly recommended by practice guidelines to decrease opioid consumption in postsurgical patients.5 These may be options to treat pain in the setting of national drug shortages; however, it is important to realize that the onset of action of these medications is much longer than IV opioids and can also lead to altered mental status in those with renal dysfunction.
Although the SCCM guidelines recommend a light sedation strategy with nonbenzodiazepine infusions (i.e., propofol and dexmedetomidine) in most ICU patients, many patients diagnosed with COVID-19 have deeper sedation requirements. Consequently, there has been a resurgence in the usage of benzodiazepine infusions.5,11 While this trend has been partially potentiated by drug shortages, COVID-19 patients may have acute lung injury that requires more aggressive ventilator strategies, thereby necessitating the need for deeper sedation for patient compliance. Additionally, the risk of a malpositioned endotracheal tube, either by self-extubation or accidental misplacement, would not only lead to poor patient outcomes but could also expose healthcare workers to COVID-19.
Patients who may require neuromuscular blocking agents (NMBAs) in ARDS to facilitate ventilator synchrony will also require deep sedation. Traditionally, when light sedation is targeted, either propofol or dexmedetomidine is utilized (TABLE 1). However, dexmedetomidine would not be an appropriate choice in patients requiring deep sedation or those who are on NMBAs, and it may also lead to hypotension and bradycardia given its mechanism of action as an alpha-2 agonist.5
Sedative Options in COVID-19
Dexmedetomidine would be a viable option in patients able to tolerate light sedation given its opioid-sparing effects, which would help mitigate ongoing shortages. The side-effect profiles of both agents require careful assessment in patients with COVID-19. Propofol can cause hypertriglyceridemia as it is a 10% fat emulsion–containing product; triglycerides should be trended while patients remain on propofol.12 Once triglycerides are greater than 500 mg/dL, nonpropofol sedation strategies should be considered. However, patients with COVID-19 may present with a secondary hemophagocytic lymphohistocytosis (HLH)-type picture with severe hypertriglyceridemia.13 HLH is the result of excessive immune activation and inflammation due to the absence of downregulated activated macrophages and lymphocytes, leading to tissue destruction and high mortality rates. Thus, triglycerides need to be more carefully monitored in this patient population, and clinicians should consider a lower threshold for transitioning to a nonpropofol sedation strategy.
Benzodiazepines are less commonly utilized for sedation given the concern for increased risk of delirium and ICU LOS.5 However, with ongoing national shortages and the potential need for deeper sedation, benzodiazepines utilization has become more frequent. Lorazepam continuous infusions are not typically employed in the ICU population due to the propylene glycol additive, which can lead to a high anion gap metabolic acidosis and renal failure. If a benzodiazepine continuous infusion is needed, midazolam is more commonly given. Midazolam may lead to prolonged sedation when used for long periods of time and will accumulate in patients with renal or hepatic dysfunction, heart failure, or obesity. Providers must be cognizant of midazolam’s active metabolite and potential for prolonged sedation even when a continuous infusion has been discontinued.
As with analgesia, drug shortages have forced clinicians to consider alternative sedation strategies. Likely one of the most effective and practical ways to limit continuous infusions is by utilizing oral dosage forms or intermittent doses of sedatives. For example, agents such as diazepam or lorazepam could be used intermittently to minimize continuous infusion–sedation requirements. Clonidine, an alpha-2 agonist, has some data for use as sedation in an ICU setting and might be appropriate to consider in hemodynamically stable patients.14,15
Ketamine is an agent that has a variety of uses in critical illness, including acute pain management, postoperative analgesia, refractory status epilepticus, and adjunctive sedation.7,16 Given its analgesic properties, it may also be opioid sparing, which would also be beneficial in the setting of drug shortages.17 There are several considerations for using ketamine in this patient population. Although ketamine is associated with hypertension and tachycardia, it may also decrease myocardial function in some subsets of critically ill patients, including those with septic shock.18 Ketamine may also be considered for usage in targeting deep sedation; however, logistical concerns then arise. When infused continuously at higher doses, the limitation starts to become the amount of volume being infused, particularly if patients are unable to keep up with their fluid balance. Liberal fluid management strategies in patients with acute lung injury may be associated with increased ventilator days, days with central nervous system failure, and days in the ICU, so fluid balance is a paramount concern in these patients.19
Volatile gases, including desflurane, isoflurane, and sevoflurane, have also garnered some interest as potential contingency sedation options. These agents are typically used as general anesthesia in patients undergoing surgery, and there is very limited evidence for their usage in the ICU, especially for longer periods of time (>24-48 hours).20-22 Other limitations include the need for special-delivery devices and lack of experience with these agents outside of the operating room. Although uncommon, clinicians should be aware of the potential for patients developing malignant hyperthermia with these inhaled anesthetics.7
NMBAs in Acute Respiratory Distress Settings
NMBAs have several proposed benefits in the setting of ARDS. They improve hypoxemia by decreasing inflammation, increasing alveolar recruitment, and improving synchrony between the patient and the ventilator.23-26 Currently, clinical practice guidelines allow for consideration of NMBAs early in ARDS (within 48 hours) for patients with a PaO2/FiO2 <150 mm Hg.27,28 These recommendations are based primarily on the findings of the ARDS et Curarisation Systematique (ACURASYS) trial in which ARDS patients with PaO2/FiO2 <150 mm Hg received cisatracurium at a flat dose of 37.5 mg/hour for 48 hours.
Compared with placebo, cisatracurium was associated with a statistically significant decrease in adjusted 90-day mortality (P = .04).23 NMBAs in ARDS were recently evaluated in another randomized, controlled trial, Re-evaluation of Systemic Early Neuromuscular Blockade (ROSE). This trial again included patients with ARDS and PaO2/FiO2 <150 mm Hg, assigning patients to either 48-hour continuous infusions of flat-dose cisatracurium (37.5 mg/hour) or placebo.29 In contrast to ACURASYS, ROSE did not find a difference in 90-day mortality. However, there are several significant differences to note between ACURASYS and ROSE, including trial design (double-blinded vs. unblinded), positive-end expiratory pressures (³5 cm H2O vs. ³8 cm H2O), average time to enrollment (15-18 vs. 6.8-8.2 hours), prone positioning (28-29% vs. 15.8%), all respectively, and the control arm of ROSE targeted lighter sedation. All of these differences could explain the discrepancy between the findings and must be considered when comparing the two.
Drug shortages complicate neuromuscular blocking strategies as well. Both ACURASYS and ROSE used flat-dose cisatricurium infusions at 37.5 mg/hour. ROSE used this strategy to replicate the protocol ACURASYS utilized, but it is important to note that ACURASYS chose a flat dose in order to maintain blinding. The authors selected that dose based on prior studies that showed two patients out of 92 total patients evaluated required doses as high as 37.5 mg/hour in order to achieve train-of-four (TOF) responses of zero.24,25,30 Accordingly, applying a weight-based strategy titrated to TOF would likely conserve drug.31 Interestingly, ROSE allowed for intermittent boluses of NMBAs in their control arm, which has led some clinicians to trial intermittent boluses of NMBAs prior to or in lieu of a continuous infusion of an NMBA, which would also reduce drug utilization.29
Conclusion and Pharmacist Involvement
Given the current drug shortages, pharmacists are in a unique position to help reduce drug utilization. Additionally, pharmacists are also essential at the bedside for patients requiring NMBAs given that it is absolutely essential to obtain and maintain deep sedation throughout the duration of NMBA. Neuromuscular blockade in conjunction with deep sedation presents additional risk for adverse effects that pharmacists are equipped to monitor. Since patients are immobilized, there is the potential risk for increased rates of venous thromboembolism.28 ICUAW is another potential complication, resulting from atrophy and immobility, as well as concomitant use of other agents that may potentiate it, including corticosteroids (which are also increasingly used for COVID-19 ARDS) and aminoglycosides.32 Critically ill patients with COVID-19 are at particularly high risk for developing delirium secondary to immobility and deep sedation as well as multiorgan system failure, social factors (e.g., isolation), and prolonged mechanical ventilation.33 Often overlooked are the long-term effects of critical illness. Critically ill patients with COVID-19 will likely face long-term recovery from physical and cognitive derangements that may significantly impact their quality of life.34 Pharmacists are key in helping limit unnecessary exposure to analgesics, sedation, and paralysis while monitoring for delirium, ICUAW, and venous thromboembolism.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl JMed. 2020;382:1708-1720.2. Centers for Disease Control and Prevention, U.S. Department of Health & Human Services. CDC COVID Data Tracker. covid.cdc.gov/covid-data-tracker/. Accessed December 1, 2020.
3. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198.
4. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574-1581.
5. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
6. Roan JP, Bajaj N, Davis FA, Kandinata N. Opioids and chest wall rigidity during mechanical ventilation. Ann Intern Med. 2018;168(9):678.
7. Ammar MA, Sacha GL, Welch SC, et al. Sedation, analgesia, and paralysis in COVID-19 patients in the setting of drug shortages. J Intensive Care Med. 2021;36(2):157-174.
8. Muellejans B, Lopez A, Cross MH, et al. Remifentanil versus fentanyl for analgesia-based sedation to provide patient comfort in the intensive care unit: a randomized, double-blind controlled trial. Crit Care. 2004;8(1):R1-R11.
9. Egan TD, Lemmens HJ, Fiset P, et al. The pharmacokinetics of the new short-acting opioid remifentanil in healthy adult male volunteers. Anesthesiology. 1993;79(5):881-892.
10. Ginsberg B, Sinatra RS, Adler LJ, et al. Conversion to oral controlled-release oxycodone from intravenous opioid analgesic in the postoperative setting. Pain Med. 2003;4(1):31-38.
11. Chanques G, Constantin JM, Devlin JW, et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020;46:2342-2356.
12. Diprivan (propofol injection, emulsion) [package insert]. Lake Zurich, IL: Fresenius Kabi USA, LLC; 2020.
13. Lima R, Filho CC, Filho CMF, et al. Hemophagocytic syndrome and COVID-19. Respir Med Case Rep. 2020;31:101162.
14. Kariya N, Shindoh M, Nishi S, et al. Oral clonidine for sedation and analgesia in a burn patient. J Clin Anesth. 1998;10(6):514-517.
15. Farasatinasab M, Kouchek M, Sistanizad M, et al. A randomized placebo-controlled trial of clonidine impact on sedation of mechanically ventilated ICU patients. Iran J Pharm Res. 2015;14(1):167-175.
16. Schwenk ES, Viscusi ER, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):456-466.
17. Patanwala AE, Martin JR, Erstad BL. Ketamine for analgosedation in the intensive care unit: a systematic review. J Intensive Care Med. 2017;32(6):387-395.
18. Lippmann M, Appel PL, Mok MS, Shoemaker WC. Sequential cardiorespiratory patterns of anesthetic induction with ketamine in critically ill patients. Crit Care Med. 1983;11(9):730-734.
19. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575.
20. Sackey PV, Martling CR, Granath F, Radell PJ. Prolonged isoflurane sedation of intensive care unit patients with anesthetic conserving device. Crit Care Med. 2004;32(11):2241-2246.
21. Mesni M, Capdevila X, Bringuier S, et al. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med. 2011;37(6):933-941.
22. Spencer EM, Willatts SM, Prys-Roberts C. Plasma inorganic fluoride concentrations during and after prolonged (greater than 24 h) isoflurane sedation: effect on renal function. Anesth Analg. 1991;73(6):731-737.
23. Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107-1116.
24. Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749-2757.
25. Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113-119.
26. Paton WD. Mode of action of neuromuscular blocking agents. Br J Anaesth. 1956;28:470-480.
27. Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69.
28. Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44(11):2079-2103.
29. Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997-2008.
30. Papazian L, Forel JM, Roch A. Neuromuscular blockers and ARDS. N Engl J Med. 2010;363:2562-2564.
31. Hraiech S, Forel JM, Guervilly C, et al. How to reduce cistaracurium consumption in ARDS patients: the TOF-ARDS study. Ann Intensive Care. 2017;7:79.
32. Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274.
33. Kotfis K, Williams Roberson S, Wilson JE, et al. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24(1):176.
34. Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6(1):60.
To comment on this article, contact rdavidson@uspharmacist.com.






