US Pharm. 2023;48(2):HS-6-HS-12.
ABSTRACT: An infection of the liver that is caused by the hepatitis C virus (HCV) is a dangerous chronic infection that can lead to life-threatening complications. There are new medications called combination direct-acting antivirals that can be utilized for HCV. This article will review these agents and explain the benefits and drawbacks, when to use them, and general medication information. It is crucial for pharmacists to be up to date on these new medications because they are in a unique position to help educate patients with HCV infection to improve overall outcomes.
Hepatitis C virus (HCV) is a dangerous infection of the liver that can lead to life-threatening complications, including cirrhosis.1 HCV is a bloodborne virus that is typically transmitted through exposure to blood from unsafe practices, such as sharing needles or unsterile blood draws, but it can occur from other causes.1 HCV infects many people worldwide. Globally, it is estimated that approximately 58 million people are living with chronic HCV infection. Every year, a projected 1.5 million new infections will occur.1 In the United States, the prevalence of chronic HCV infection was about 2.4 million people during 2013-2016.2 In the U.S., there were also an estimated 57,500 acute HCV cases in 2019. The World Health Organization stated that in 2019, approximately 290,000 people who had HCV infection died, mostly from cirrhosis and hepatocellular carcinoma.3 Considering the alarming number of individuals who not only are affected but die from HCV infection, it is important to understand how to combat this virus and not only help extend patients’ lives but also improve their quality of life.
Types of HCV Infections
There are two different classifications of HCV infections: acute and chronic. Acute HCV presents as a discrete onset of flu-like symptoms, including nausea, fever, and malaise.3 These symptoms may be accompanied by jaundice or elevated serum aminotransferase levels based on laboratory confirmation.3 Chronic HCV presents as a persistent infection that is detected by HCV RNA appearing in the blood for 6 or more months that causes liver inflammation with progressive fibrosis. Chronic HCV can result in hepatocellular carcinoma, cirrhosis, and end-stage liver disease.1
Further classification of HCV is through hepatitis C genotypes. A hepatitis C genotype is a strain of HCV. There are six genotypes of hepatitis C around the world, with different regions having more common genotypes than other regions. In the U.S., genotype 1 is the most common; other common genotypes are 2 and 3.4 A patient’s genotype is typically tested once, as it will not change over time and can help clinicians guide pharmacologic treatment.
Risk Factors
HCV infections can result from several causes, and the risk factors are associated with certain activities. Examples of activities that increase risk of HCV include substance abuse, hemodialysis, surgical procedures, HIV infections, alcohol abuse, and sexual exposure.3
Signs and Symptoms
HCV can present through many different signs and symptoms. It is crucial to a patient’s overall health to recognize these signs and symptoms and talk with their doctor quickly. Once signs and symptoms have been observed, different testing may be completed to determine the diagnosis of HCV infection. Examples of signs and symptoms for HCV infections include jaundice, loss of appetite, liver pain, hepatosplenomegaly, spider nevus, myalgia, fatigue, alcoholic stool (clay- or light-colored stools), arthralgia, and erythema.3
Diagnostic Tools
A hepatitis C antibody measurement is commonly performed to assess the amount of antibodies to the HCV in the blood. Other tests and laboratory measurements that may be assessed include hepatitis C nucleic acid assay, HCV genotype determination, alanine aminotransferase measurement, or a complete blood count. Not only are these tests and laboratory values collected, but prior to initiation of medications, it is important to test all patients for evidence of current or previous hepatitis B virus exposure. There is no vaccination that can prevent hepatitis C, so the best way to prevent this infection is through avoiding behaviors that can spread it. Since HCV is a blood-borne virus, it is typically transmitted through reuse of needles and syringes, inadequate sterilization of medical equipment, transfusion of unscreened blood, or shared medical equipment. Transmission can also occur through an infected mother passing the infection to the fetus.
Treatment
If prevention does not work, there are treatment options available. The American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) released an update in 2019 to the recommendations for testing, managing, and treating HCV infection.5 A new infection may or may not require treatment; some individuals mount an immune response that can clear the infection. If HCV becomes chronic, treatment is warranted. The goal of HCV therapy is to achieve undetectable levels of HCV RNA or a sustained virologic response.6 The AASLD and the IDSA guidelines recommend antiviral treatment for all adults with acute or chronic HCV infection, except those with a short life expectancy who cannot be remediated by therapy, liver transplant, or other direct therapy.5
There are medications that can cure the infection within 8 to 24 weeks, including combination DAAs.1 Combination DAAs are the most common treatment for hepatitis C and can cure the infection in 90% to 97% of cases.7 These medications are all taken orally, and they include ledipasvir/sofosbuvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pibrentasvir. The main indicator for each medication is based on the coverage of the patient’s HCV genotype. For example, ledipasvir/sofosbuvir and elbasvir/grazoprevir do not cover genotype 2, so if a patient has genotype 2 of HCV these would not be selected over other options, such as glecaprevir-pibrentasvir, which does cover genotype 2.7 These drugs work by attacking the HCV directly. The recommended regimens based on the guidelines for chronic HCV infection include glecaprevir/pibrentasvir and sofosbuvir/velpatasvir.5 An 8-week course of the daily, fixed-dose combination of glecaprevir/pibrentasvir is recommended in children and adolescents aged 3 years or older.5
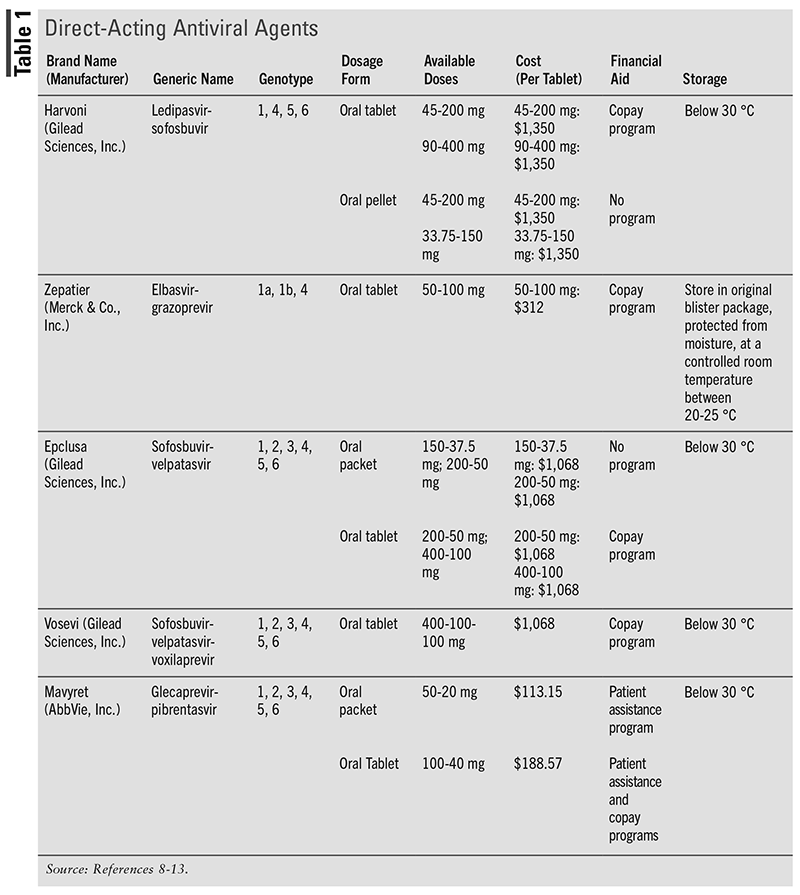
The primary recommendation for treating HCV infection is DAAs, which are one of the top 10 most common therapies offered by specialty pharmacies. The drawback to DAAs is that they are extremely expensive.7 As mentioned earlier, genotype can determine treatment options. Each medication covers certain genotypes, so it is important to test patients to determine which medication will work best for them. TABLE 1 and the following section describes all of the DAAs, including the brand name, generic name, dosage form, dose, genotype coverage, cost, financial aid, and storage.
Barrier to Treatment: Medication Cost
A major concerning issue surrounding HCV is access to medications. The most commonly used medications include direct-acting antiviral (DAA) agents.8 These agents are highly effective at clearing the infection in over 90% of people. There are now combination therapies of DAAs that are being utilized as well that can be beneficial for different types of HCV. These DAAs are newer classes of drug therapies that are considered to be of high cost and high complexity. Medications that are high cost and high complexity are typically called specialty drugs or specialty pharmaceuticals. Across the U.S., one of the most common therapy areas of need for specialty medications is hepatitis, which includes these DAAs.7 Considering these therapy options and with cost a major barrier, it is important to fully utilize pharmacists to the best of their ability to help patients with a multidisciplinary approach.
Ledipasvir/Sofosbuvir
Harvoni is the brand name of ledipasvir in combination with sofosbuvir that was FDA approved in in 2014.14 Adult dosing for ledipasvir/sofosbuvir is for genotypes 1, 4, 5, and 6. There are different dosing strategies that providers will utilize for different patient situations, including decompensated cirrhosis, HIV infection, post–liver transplant, treatment-experienced, or treatment-naive. This medication can be taken with or without food, but it is important not to chew and to separate the administration of antacids and ledipasvir/sofosbuvir by 4 hours. It is important to be aware that bradycardia may occur if the patient is also taking amiodarone or beta-blockers or has underlying cardiac comorbidities. Common adverse effects include fatigue and neurologic symptoms, such as asthenia and headache. Serious adverse effects include hepatic symptoms, such as liver failure or hepatitis B virus reactivation, and psychiatric symptoms, such as suicidal behavior.9
The black box warning indicates that patients must be tested to determine any previous or current exposure to hepatitis B virus prior to initiating ledipasvir/sofosbuvir due to the potential of reactivation.9 There are several drug-drug interactions, so it is important for providers to understand which medications a patient is currently taking to determine any drug-drug interactions that may occur. It is recommended to stop taking St. John’s wort prior to starting ledipasvir/sofosbuvir to avoid this interaction.9 There are multiple DAAs that may be options for this patient population. Out of three large, multicenter, randomized studies, there were 1,952 subjects and only 29 HCV genotype 1a and 8 HCV genotype 1b with virological failures.14 Studies of ledipasvir/sofosbuvir show that it is effective, even though it was one of the first combination DAAs approved by the FDA.9
Elbasvir/Grazoprevir
Zepatier is the brand name of elbasvir in combination with grazoprevir that was FDA approved in 2016. Adult dosing for elbasvir/grazoprevir is for genotypes 1 and 4. There are also different dosing strategies providers will utilize for different patient situations, similar to those employed with ledipasvir/sofosbuvir. Different patient situations that may dictate treatment dosing and frequency may include treatment failure with other medications, such as peginterferon alfa and ribavirin, HIV infection, treatment-experienced, or treatment-naive. This medication can be taken with or without food. There are several medications that are contraindicated if taking elbasvir/grazoprevir, including organic anion transporting polypeptides 1B1/3, CYP3A4 inducers, efavirenz, and ribavirin. Elbasvir/grazoprevir is also contraindicated in pregnancy. An important precaution to be aware of is hepatic decompensation and increases in ALT. Common adverse effects include fatigue, headache, and nausea. Serious adverse effects include hepatic symptoms such as liver failure or hepatitis B virus reactivation.
Per the black box warning, patients must be tested to determine any previous or current exposure to hepatitis B virus prior to initiating elbasvir/grazoprevir due to the potential of reactivation. There are several drug-drug interactions, so it is crucial for providers to understand which medications a patient is currently taking to determine any drug-drug interactions that may occur.10 Considering the other DAAs that are available and that elbasvir/grazoprevir is not recommended first line by the guidelines, it is reasonable to consider it an option for patients after failure of other therapy. A study published in 2015 showed that 12 weeks of grazoprevir and elbasvir plus ribavirin provided a promising treatment option if patients experienced a failure of triple therapy that included an earlier generation protease inhibitor.15
Sofosbuvir/Velpatasvir
Epclusa is the brand name of sofosbuvir in combination with velpatasvir that was approved by the FDA in 2016.11 Adult dosing for sofosbuvir/velpatasvir is for genotypes 1, 2, 3, 4, 5, and 6.11 There are different dosing strategies that providers will utilize for specific patient situations, which may include transplantation of liver, HIV infection, post–liver transplant, or decompensated cirrhosis. This medication can be taken with or without food, but it is important not to chew and to not take it within 4 hours of an antacid or proton pump inhibitor. A precaution to be aware of is bradycardia, which may occur if the patient is also taking amiodarone or beta-blockers or has underlying cardiac comorbidities. Common adverse effects include fatigue, headache, and nausea. Serious adverse effects include hepatic symptoms, such as liver failure or hepatitis B virus reactivation, and bradyarrhythmia.11
The black box warning for sofosbuvir/velpatasvir indicates that patients must be tested to determine any previous or current exposure to hepatitis B virus prior to initiating sofosbuvir/velpatasvir due to potential of reactivation. There are several drug-drug interactions, so it is crucial for providers to understand which medications a patient is currently taking to determine any medication interactions that may occur. A common drug-drug interaction of sofosbuvir/velpatasvir is with St. John’s wort. Concurrent use of sofosbuvir/velpatasvir and P-glycoprotein inducers may decrease the sofosbuvir efficacy.11 Storage is an important aspect for ensuring treatment is going to be efficacious. Sofosbuvir/velpatasvir is one of the recommended regimens per the guidelines from the AASLD and IDSA.5 There have been multiple clinical trials to show the efficacy of sofosbuvir/velpatasvir, including one open-label trial in Japan. At the time, Japan had no treatment options for HCV-infected patients with decompensated cirrhosis. Utilizing sofosbuvir/velpatasvir for 12 weeks showed that it is highly effective and well tolerated for patients with HCV and decompensated cirrhosis.16
Sofosbuvir/Velpatasvir/Voxilaprevir
Vosevi is the brand name of the combination sofosbuvir/velpatasvir/voxilaprevir, which was FDA approved in 2017. Adult dosing for sofosbuvir/velpatasvir/voxilaprevir is for genotypes 1, 2, 3, 4, 5, and 6. There are different dosing strategies providers will utilize for various patient situations that may include cirrhosis or previously treated with nonstructural protein 5A inhibitors. This medication should be taken with food. Vosevi is contraindicated with concomitant use with rifampin. An important precaution to be aware of is bradycardia may occur if a patient is also taking amiodarone or beta-blockers or has underlying cardiac comorbidities. Another important precaution is that hepatic decompensation or hepatic impairment can occur while taking sofosbuvir/velpatasvir/voxilaprevir. Common adverse effects include nausea, diarrhea, headache, and fatigue. Serious adverse effects include hepatic symptoms, such as liver failure or hepatitis B virus reactivation and bradyarrhythmia.12
The black box warning to be aware of is that patients must be tested to determine any previous or current exposure to hepatitis B virus prior to initiating sofosbuvir/velpatasvir/voxilaprevir due to potential reactivation. There are several drug-drug interactions, so it is crucial for providers to be aware of medications a patient is currently taking to determine any drug-drug interactions that may occur.12 When choosing different DAAs, this agent may be a great option when treatment with another DAA failed. A clinical trial in 2017 noted that patients who are chronically infected with HCV and who have not sustained virologic response after treatment have limited options for retreatment. This study showed that after 12 weeks, taking sofosbuvir/velpatasvir/voxilaprevir provided high rates of sustained virologic response among patients in whom treatment with a DAA previously failed.17
Glecaprevir/Pibrentasvir
Mavyret is the brand name of glecaprevir in combination with pibrentasvir, which was approved by the FDA in 2017. Adult dosing for glecaprevir/pibrentasvir is for genotypes 1, 2, 3, 4, 5, and 6. There are different dosing strategies for different patient situations, which may include patients with cirrhosis or who were previously treated with NS5A inhibitors. This medication should be taken with food. Glecaprevir/pibrentasvir is contraindicated in patients with moderate or severe hepatic impairment and in patients who take atazanavir or rifampin. An important precaution to be aware of is hepatic decompensation or failure. Common adverse effects of glecaprevir/pibrentasvir include fatigue, headache, and nausea. Serious adverse effects include hepatic symptoms, such as liver failure or hepatitis B virus reactivation.13
The black box warning to be aware of is that patients must be tested to determine any previous or current exposure to hepatitis B virus prior to initiating glecaprevir/pibrentasvir due to potential of reactivation. In addition, there are several drug-drug interactions, so it is crucial for providers to understand patients’ current medication regimens to determine any drug-drug interactions that may occur. Storage is also an important aspect for ensuring treatment efficacy. Glecaprevir/pibrentasvir is another option that is recommended by the AASLD and IDSA.5 There are multiple studies observing high efficacy and safety of glecaprevir/pibrentasvir, and it was shown to be well-tolerated in chronic HCV–infected patients with compensated cirrhosis, immunodeficiency virus coinfection, end-stage renal disease, liver or renal transplant or recent drug use, and in adolescents. These populations encompass common diseases and situations, so this may be a great option for patients who have other disease states.13
The Role of the Pharmacist
Considering the financial impact and other barriers of obtaining medication, such as availability of these new combination DAAs, there is a high percentage of patients who are not receiving treatment. In 2019, there were approximately 58 million persons living with an HCV infection globally. Out of those 58 million, there were an estimated 15.2 million patients who knew about the diagnosis, and only 9.2 million of those individuals were being treated with DAAs.3 It is crucial for patients who suspect they may have HCV infection to talk with their doctor and be diagnosed and treated as quickly as possible. Not only are doctors critical in the management of HCV, but pharmacists are in a unique position to help patients manage this infection.
Considering cost barriers, pharmacists can also work with providers and patients to determine the most cost-effective treatment regimen as well as aid patients in applying for patient-assistance programs or obtaining copay cards. Pharmacists can assess patients’ genotypes and help make recommendations for treatment options that can be efficacious for the specific type of HCV. One, if not the most important, role of a pharmacist in helping patients manage their hepatitis C infection is counseling patients about the medications that are available. Discussing duration, frequency, adverse effects, drug interactions, and more is needed for successful treatment outcomes. Pharmacists are in a unique position to help this patient population and should be utilized in different clinical settings to provide this care.
Conclusion
HCV is a life-threatening infection. Newly emerging treatment is life-changing for patients who get HCV. Combination DAAs are not the only treatment option for HCV infections; however, they are utilized despite the financial barrier due to their safety profiles and effectiveness. Pharmacists can play a unique role in helping manage these medications and counseling patients who have HCV, which can be very beneficial for this patient population. Overall, fully utilizing the available treatment resources portends a promising future for those infected with HCV.
REFERENCES
1. CDC. Hepatitis C questions and answers for health professionals. 2020. www.cdc.gov/hepatitis/hcv/hcvfaq.htm#section4. Accessed January 20, 2023.
2. Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69:1020-1031.
3. World Health Organization. Hepatitis C. 2022. www.who.int/news-room/fact-sheets/detail/hepatitis-c#:~:text=Globally%2C%20an%20estimated%2058%20million,with%20chronic%20hepatitis%20C%20infection. Accessed January 20, 2023.
4. U.S. Department of Veteran Affairs. Hepatitis C genotype. 2019. www.hepatitis.va.gov/hcv/patient/diagnosis/labtests-hepatitisC-genotype.asp. Accessed January 20, 2023.
5. Ghany MG, Morgan TR. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686-721.
6. Rabaan AA, Al-Ahmed SH, Bazzi AM, et al. Overview of hepatitis C infection, molecular biology, and new treatment. J Infect Public Health. 2020;13(5):773-783.
7. Definitive Healthcare. Top specialty pharmacy therapy areas. 2022. www.definitivehc.com/blog/top-specialty-pharmacy-therapy-areas. Accessed January 20, 2023.
8. Healthline. A full list of hepatitis C medications: Epclusa, Harvoni, Zepatier, and more. 2022. www.healthline.com/health/hepatitis-c/full-medication-list. Accessed January 20, 2023.
9. Harvoni (ledipasvir and sofosbuvir) product information. Foster City, CA: Gilead Sciences, Inc; 2020.
10. MerckHelps. Zepatier. Merck & Co., Inc. 2023. www.merckhelps.com/zepatier. Accessed January 20, 2023.
11. Epclusa (sofosbuvir and velpatasvir) product information. Foster City, CA: Gilead Sciences, Inc; 2022.
12. Vosevi (sofosbuvir, velpatasvir, and voxilaprevir) product information. Foster City, CA: Gilead Sciences, Inc; 2019.
13. Mavyret (glecaprevir and pibrentasvir) product information. North Chicago, IL: AbbVie, Inc; 2021.
|14. Gritsenko D, Hughes G. Ledipasvir/sofosbuvir (Harvoni): improving options for hepatitis C virus infection. P T. 2015;40(4):256-276.
15. Forns X, Gordon SC, Zuckerman E, et al. Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J Hepatol. 2015;63(3):564-572.
16. Takehara T, Sakamoto N, Nishiguchi S, et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: an open-label phase 3 trial. J Gastroenterol. 2019;54(1):87-95.
17. Bourlière M, Gordon SC, Flamm SL, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376:2134-2146.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






