US Pharm. 2019;44(8):17-23.
ABSTRACT: In recent years, testosterone replacement therapy (TRT) has received significant media attention, and the rate of testosterone use has increased notably. A reported association between testosterone use and increased occurrence of myocardial infarction and stroke prompted the FDA to issue a safety bulletin in 2014. Clinical hypogonadism is the only FDA-approved indication for TRT in men; it is not approved to treat age-related low testosterone. Although it is not indicated, TRT is often recommended to improve sexual function, bone density, body composition, muscle strength, mood, behavior, and cognition. The literature on the effectiveness of TRT for various conditions is largely mixed; therefore, current data on appropriate and potentially inappropriate use are important for pharmacists to keep abreast of and discuss with patients.
Recently, the use of testosterone replacement therapy (TRT) has received a lot of media attention. Although its use is growing, there is much debate regarding TRT’s risks and benefits.1 From 2008 to 2012 in the United States, spending on TRT increased from $1 billion to $2 billion, and from 2003 to 2013 there was a fourfold increase in the rate of TRT in men aged 18 to 45 years.2 In 2013 and early 2014, two studies reported an association between TRT and increased occurrence of myocardial infarction and stroke, prompting the FDA to issue a safety bulletin on January 31, 2014.3 This article will discuss appropriate TRT use, available formulations and cost, side effects, trends, and the pharmacist’s role in patient education, including counseling points.
Physiology
Testosterone, which is essential for the development and maintenance of organs and physiological functions in males, has many biological effects. In males, this hormone is produced by Leydig cells in the testes in response to luteinizing hormone from the pituitary gland. In females, testosterone is made in the ovaries and adrenal glands and is an essential precursor to estrogen; it is associated with mood, sexual function, and bone health.4,5
Indications and Other Uses
TRT in males with low concentrations of testosterone is controversial because this condition is often a normal sign of aging; testosterone concentrations in men naturally begin to decline at age 40 years, at an average of 1% to 2% per year.5 Clinical hypogonadism, however, is an approved indication for TRT and is the only FDA-approved indication for TRT in men. The American Association of Clinical Endocrinologists (AACE) defines male hypogonadism as a decrease in testicular function (sperm or testosterone production) accompanied by signs or symptoms.6 In women, additional FDA-approved indications include vasomotor symptoms of menopause (using a combination of esterified estrogens and methyltestosterone) and breast cancer. Despite having only the abovementioned FDA-approved indications, TRT is often recommended by prescribers—based on a presumption of low testosterone—for sexual function, bone density, body composition, muscle strength, mood, behavior, and cognition. Two areas of use that are beyond the scope of this article are TRT in sports—an ongoing controversy—and in female-to-male transgender patients.
Diagnosis
Based on a lack of data regarding screening tools, the Endocrine Society currently advises against routine screening of men for low testosterone.7 However, it does recommend that men with symptoms or signs of testosterone deficiency and consistently low serum testosterone concentrations be tested for hypogonadism.7 The healthcare provider should confirm that the patient has low testosterone concentrations on at least two occasions and should evaluate associated signs and symptoms via questionnaire. Commonly used assessments for diagnosis of clinical hypogonadism include the Androgen Deficiency in the Aging Male questionnaire and the Aging Males’ Symptoms scale. Confirmatory diagnosis is made via total testosterone, luteinizing hormone, and follicle-stimulating hormone concentrations.7 Circulating testosterone concentrations vary throughout the day, peaking in the early morning; therefore, fasting concentrations should be obtained between 8 am and 10 am.6-8
Conditions
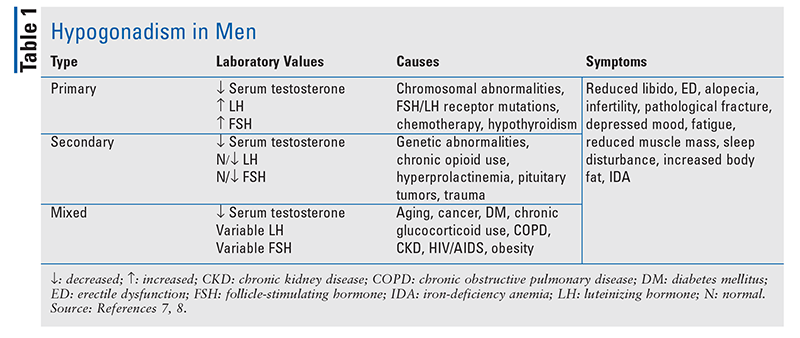
Hypogonadism: Hypogonadism is classified as primary, secondary, or mixed. In males, primary hypogonadism results from dysfunction of the testes; secondary hypogonadism results from dysfunction of the pituitary or hypothalamus. See TABLE 1 for a summary of laboratory values, causes, and symptoms of hypogonadism. Although there is no universal laboratory definition of hypogonadism, most laboratory reference ranges for males report total testosterone concentrations <300 ng/dL as diagnostic, with a reference range of 300 ng/dL to 1,000 ng/dL.6-8
Menopause-Associated Vasomotor Symptoms: Resulting from thermoregulatory dysfunction, the vasomotor symptoms of menopause (e.g., hot flashes) are associated with poor sleep, irritability, poor concentration, and decreased quality of life.9 Hormone therapy with estrogen and progesterone is recommended for symptom management; however, treatment failure can lead to the recommendation to add TRT.10 Although evidence of efficacy is minimal and long-term studies are lacking, TRT may be an appropriate means of management in certain patients.
Breast Cancer: The National Comprehensive Cancer Network guidelines state that androgen therapy “may be useful in certain circumstances” in postmenopausal women with human epidermal growth factor receptor 2 (HER2)–negative, hormone receptor–positive breast cancer.11 In breast and endometrial cancer, androgens may slow or prevent tumor progression when they are combined with usual therapy options.12
Male Sexual Function: Despite mixed study results regarding increased libido and improved erectile function, TRT is often prescribed. In a systematic review of 47 studies assessing sexual function, 23 studies reported beneficial effects for TRT and 24 studies found no improvement in any sexual-function endpoint.13 In the studies with positive outcomes, the effect of testosterone was smaller than with phosphodiesterase type 5 inhibitors.14 The American Urological Association states that although TRT can increase sexual interest, it has no significant influence on erectile function in men with normal testosterone concentrations.2
Female Sexual Function: TRT has been shown to be effective for improving libido, sexual desire, arousal, sexual frequency, and sexual satisfaction in women.15,16 In most trials, women receive concurrent estrogen therapy; however, recent trials have reviewed use of TRT alone in postmenopausal women.17 These benefits have also been seen in patients receiving TRT for other indications, such as mood regulation. Although no testosterone products are approved to improve female sexual function, many have been attempted, including oral pills, SC tablets, IM injections, transdermal patches, gels, creams, sprays, and sublingual drops. Dosing is typically lower than that used in men, and monitoring includes symptom management rather than laboratory evaluation.18
Osteoporosis: TRT is also used in men with poor bone density, body composition, and muscle strength. Although osteoporosis is less common in men than in women, more than 8 million men in the U.S. have osteopenia or osteoporosis.19 A low testosterone concentration reduces bone density and changes body composition. In middle-aged men with low testosterone concentrations, TRT increases bone density in the lumbar spine.20 In older men, TRT increases bone density in the spine and hip.13 There is no evidence that TRT decreases fractures or falls. The National Osteoporosis Foundation gives no recommendations for TRT use in patients with osteoporosis.19
Mood and Quality of Life: A small number of studies have reported mixed results for improved mood, vitality, and quality of life in aging men receiving TRT.13,20 Most studies used standard questionnaires to assess these factors.13,21 TRT has been demonstrated to improve symptoms of depression in men with hypogonadism and HIV/AIDS.13 National HIV/AIDS guidelines do not provide recommendations for TRT use, however.22
Cognition: TRT has been linked to effects on cognitive skills.13,23 Several small studies have had mixed results for cognition, some of them finding benefit in men with mild cognitive deficits or memory disorders such as Alzheimer’s disease (AD).13,23 However, most of these studies were short-term and had small sample sizes. Treatment guidelines for AD management do not recommend the use of TRT.24 Data are similarly scarce in women; a limited number of studies have demonstrated improvements in psychological and cognitive effects of menopause.25,26
Diabetes: Few clinical trials have been conducted on whether TRT can improve glucose control. Some small studies have found improvements in A1C.13 A 2019 article stated that testosterone deprivation has been shown to increase blood-glucose concentrations and that glucose concentrations improved after TRT initiation.27 The American Diabetes Association standards of medical care have no suggestions for TRT use.28
Risks
In 2015, as part of its advisory on cardiovascular risk, the FDA issued a safety announcement that TRT is approved only for use in men with low testosterone concentrations caused by hypogonadism and should not be used in those with age-related low testosterone.3 In the same communication, the FDA strongly cautioned prescribers that TRT use for other indications is off-label. Despite reasonable uses for TRT both on- and off-label, questions about its risk-versus-benefit profile remain. Much of the current data show mixed results.
Risks and side effects of TRT include development or acceleration of prostate or breast cancer, development or worsening of benign prostatic hyperplasia, increased risk of polycythemia, development or worsening of acne, alopecia, gynecomastia, worsening sleep apnea, increased lower urinary tract symptoms (LUTS), liver toxicity, and cardiovascular events.8,13 The greatest concerns regarding TRT involve older men because the potential benefits are less clear and the risks are greater. Because contraindications exist, it is important to obtain a thorough history and physical examination prior to initiating TRT. Absolute contraindications include breast cancer, polycythemia, prostate cancer, prostate-specific antigen (PSA) >4 ng/mL, and nodules upon digital rectal examination (DRE). Relative contraindications include hematocrit >50%, desire for fertility, severe LUTS, uncontrolled congestive heart failure, and untreated obstructive sleep apnea.7,8
Because the most commonly used testosterone preparations in women are oral, increased hepatotoxic risks are associated with the extensive first-pass metabolism of testosterone. Additional side effects in women include hirsutism, acne, voice deepening, alopecia, polycythemia, sleep apnea, and weight gain.5 These effects are often mitigated by the very low doses used to treat women.
There is a black box warning for secondary exposure of children to topical testosterone. Testosterone may be transferred to another person following skin contact with the application site; therefore, patients should strictly adhere to instructions for use in order to prevent secondary exposure. Children and women should avoid contact with application sites of men who are using topical products.
Counseling Points
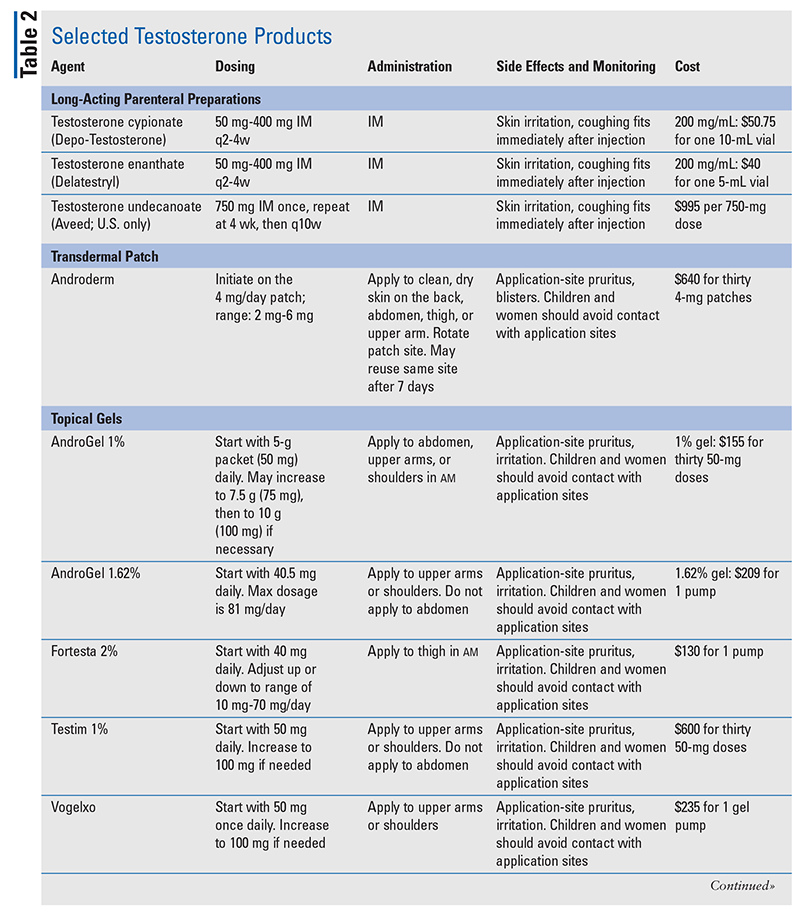
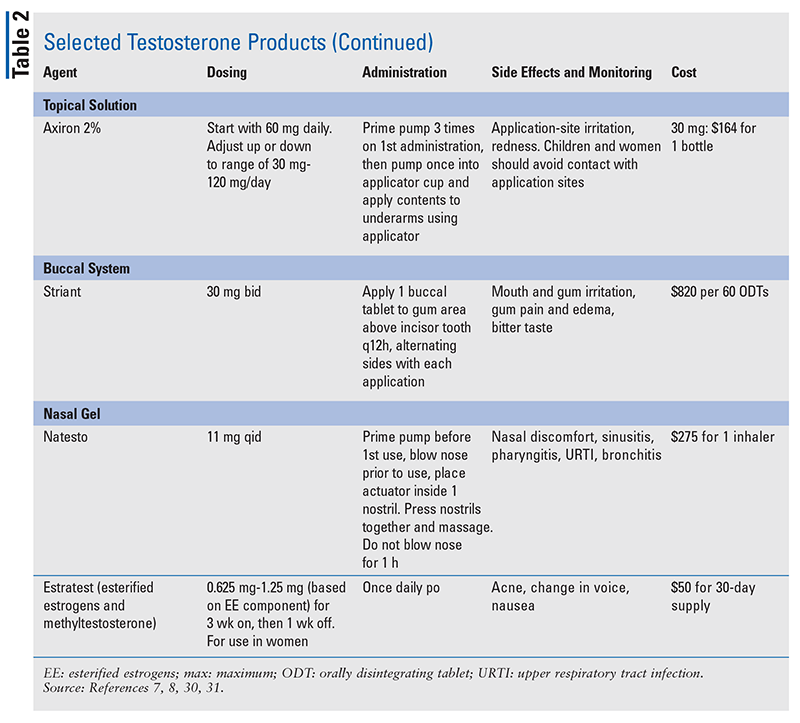
The pharmacist’s role is to educate patients about TRT. Pharmacists have an up-front opportunity to discuss TRT’s risks and benefits with patients. It is most important for patients to understand that testosterone-containing products are Schedule III substances, are approved only to treat hypogonadism, and must be used as directed by the physician. Risk Evaluation Management Strategies warnings for TRT include increased risk of heart attacks and strokes, hypertension, and pulmonary oil microembolism.3,29 See TABLE 2 for detailed information regarding individual testosterone products and specific educational points.
Monitoring
The AACE guidelines suggest that testing be performed before a patient starts using a testosterone product.6 Although there are no specific recommendations, the consensus is that testosterone concentrations be targeted to the mid-normal range of 400 ng/dL to 700 ng/dL. A history and physical examination—including DRE—and total serum testosterone, CBC, and PSA should be performed at baseline, 3 to 6 months after therapy initiation, and then annually if stable. A bone-density scan should be obtained at baseline and then annually, in addition to annual mammogram and endometrial ultrasonography in women.7,8,13
Conclusion
The literature supporting TRT is largely mixed and often controversial. However, there are many uses for TRT, some of them acceptable and others under debate. It is important for pharmacists to keep abreast of the available literature in order to provide the best education for patients.
REFERENCES
1. Miller E. Testosterone products. www.drugwatch.com/testosterone/supplements. Accessed May 28, 2019.
2. Rao PK, Boulet SL, Mehta A, et al. Trends in testosterone replacement therapy use from 2003 to 2013 among reproductive-age men in the United States. J Urol. 2017;197:1121-1126.
3. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. www.fda.gov/Drugs/DrugSafety/ucm436259.htm. Accessed May 28, 2019.
4. Ullah MI, Riche DM, Koch CA. Transdermal testosterone replacement therapy in men. Drug Des Devel Ther. 2014;8:101-112.
5. Margo K, Winn R. Testosterone treatments: why, when, and how? Am Fam Physician. 2006;73:1591-1598.
6. Petak SM, Nankin HR, Spark RF, et al. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Pract. 2002;8:440-456.
7. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715-1744.
8. Petering RC, Brooks NA. Testosterone therapy: review of clinical applications. Am Fam Physician. 2017;96:441-449.
9. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
10. Goodman NF, Cobin RH, Ginzburg SB, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause. Endocr Pract. 2011;17(suppl 6):1-25.
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines). Breast cancer. Version 1.2019. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 1, 2019.
12. Somboonporn W, Davis SR; National Health and Medical Research Council. Testosterone effects on breast: implications for testosterone therapy for women. Endocr Rev. 2004;25:374-388.
13. Cunningham GR, Toma SM. Why is androgen replacement in males controversial? J Clin Endocrinol Metab. 2011;96:38-52.
14. American Urological Association. Fifteen things physicians and patients should question. www.choosingwisely.org/societies/american-urological-association. Accessed May 28, 2019.
15. Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21:227-236.
16. Elraiyah T, Sonbol MB, Wang Z, et al. The benefits and harms of systemic testosterone therapy in postmenopausal women with normal adrenal function: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:3543-3550.
17. Testosterone therapy for menopausal women. Drug Ther Bull. 2017;55:57-60.
18. Bolour S, Braunstein G. Testosterone therapy in women: a review. Int J Impot Res. 2005;17:399-408.
19. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
20. Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177:471-479.
21. Zarrouf FA, Artz S, Griffith J, et al. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15:289-305.
22. Kaplan JE, Benson C, Holmes KK, et al. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1-207.
23. Fuller SJ, Tan RS, Martins RN. Androgens in the etiology of Alzheimer’s disease in aging men and possible therapeutic interventions. J Alzheimers Dis. 2007;12:129-142.
24. Winslow BT, Onysko MK, Stob CM, Hazlewood KA. Treatment of Alzheimer disease. Am Fam Physician. 2011;83:1403-1412.
25. Sherwin BB, Gelfand MM. Differential symptom response to parenteral estrogen and/or androgen administration in the surgical menopause. Am J Obstet Gynecol. 1985;151:153-160.
26. Wisniewski AB, Nguyen TT, Dobs AS. Evaluation of high-dose estrogen and high-dose estrogen plus methyltestosterone treatment on cognitive task performance in postmenopausal women. Horm Res. 2002;58:150-155.
27. Asih PR, Tegg ML, Sohrabi H, et al. Multiple mechanisms linking type 2 diabetes and Alzheimer’s disease: testosterone as a modifier. J Alzheimers Dis. 2017;59:445-466.
28. American Diabetes Association. Standards of medical care in diabetes 2019. www.diabetes.org. Accessed May 28, 2019.
29. FDA. Risk Evaluation and Mitigation Strategies (REMS). www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems. Accessed May 25, 2019.
30. GoodRx. www.goodrx.com. Accessed May 28, 2019.
31. Pharmacist’s letter. Comparison of testosterone products. http://pharmacistsletter.therapeuticresearch.com/home.aspx?cs=&s=PL. Accessed May 26, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.






