US Pharm. 2016;41(11) (Specialty&Oncology suppl):14-18.
ABSTRACT: Breast cancer is one of the most common cancers in American women. The high cost of brand-name breast cancer medications results in a low rate of chemoprevention of invasive breast cancer. Patient adherence is also affected by misconceptions about the efficacy of generics. Following the approval of less-expensive generic formulations of brand-name breast cancer medications, more women are using generics for prevention. Cost savings from generics have significantly increased adherence rates, and the impact of generic medications on breast cancer survival rates is expected to be significant in the long term. By making recommendations about generic versions of breast cancer medications to high-risk patients, pharmacists can play a significant role in increasing the number of women opting for breast cancer chemoprevention, thereby increasing the survival rate.
Breast cancer, one of the most common cancers diagnosed in American women, constitutes about 30% of newly diagnosed cancers in women. Since 1989, death rates from breast cancer have declined thanks to earlier detection, increased awareness, prevention, and treatment improvement. The incidence of breast cancer dropped by 7% from 2002 to 2003, owing in part to the reduced use of hormone replacement therapy. However, breast cancer remains a common threat for women; approximately 12% of women will develop invasive cancer in their lifetime. It was estimated that, in 2015, more than 2.8 million women had a history of breast cancer; 230,000 new cases of invasive breast cancer would be diagnosed; and about 40,000 U.S. women would die of breast cancer.1
Risk Assessment for Breast Cancer
Risk factors for breast cancer include modifiable and nonmodifiable risk factors.2 The modifiable risk factors include alcohol consumption, obesity, oral contraceptives, postmenopausal hormone therapy, and childbearing. To reduce their risk of breast cancer, women can avoid alcohol, exercise regularly, diet to control weight, and stop hormone therapy. Nonmodifiable risk factors, which include gender, age, genetic changes, family and/or personal history of breast cancer, race or ethnicity, dense breast tissue, certain benign breast conditions, ductal carcinoma in situ (DCIS), lobular carcinoma in situ (LCIS), menstrual periods, and previous chest radiation, cannot be changed or eliminated.2,3
To determine levels of breast cancer risk in women, clinicians have developed risk-assessment tools based on age, race or ethnicity, age at menarche, age at first live childbirth, personal history of DCIS or LCIS, number of first-degree relatives with breast cancer, smoking history, alcohol use, diet, and physical exercise. The following models are used to assess risk: the Gail model, the Gail model 2 (basis for eligibility in some breast cancer prevention trials), the Women’s Contraceptive and Reproductive Experiences (CARE) model (a modification of the Gail model for black women), and BRCA (breast cancer gene) probability tools (TABLE 1).
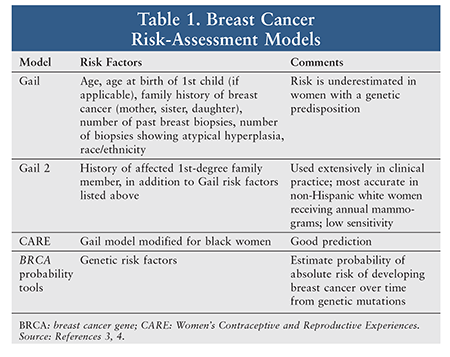
The Gail model, a computerized National Cancer Institute risk-assessment tool that used data from the Breast Cancer Detection Demonstration Project (BCDDP), was developed in 1989 to predict a woman’s absolute risk of developing breast cancer over a defined age interval. The Gail model score is based on age, age at birth of first child (if applicable), family history of breast cancer (mother, sister, daughter), number of past breast biopsies, number of biopsies showing atypical hyperplasia, and race or ethnicity. The revised model (Gail model 2) includes the risk factor of an affected first-degree family member. The Gail model 2 has been used extensively in clinical practice for breast cancer prevention to determine if a patient is at high risk.3,4 The Gail model 2 is most accurate for non-Hispanic white women receiving annual mammograms; it is less accurate in those not receiving annual mammograms and in those of different racial or ethnic groups. In addition, its sensitivity is low because its parameters are based on the general population.3,4 The computer program for the Gail model prompts users to enter input data about individual risk factors and provides a printout showing the 5-year projected breast cancer risk and the lifetime risk. The Gail model does not estimate risk in women with a personal history of breast cancer, DCIS, LCIS, or prior thoracic radiation to the chest for treatment of Hodgkin lymphoma. In addition, this model does not include the risks of current or previous use of hormone therapy, breast density, paternal family history, or maternal family history of breast cancer. As a result, the GAIL model may underestimate breast cancer risk, especially in women with a genetic predisposition, such as BRCA1 or BRCA2.4
The CARE model, which is used to estimate breast cancer risk in black women, was developed using data from a large case-control study of black women who participated in the CARE study. According to the Women’s Health Initiative cohort, the CARE model shows a good correlation between the predicted number and the observed number of breast cancers in black women.3
BRCA probability tools include the following models: BRCAPRO, Myriad I and II, Manchester scoring system, Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm, and Ontario Family History Assessment Tool. These models use genetic risk factors to estimate the absolute risk of breast cancer development over time. These tools use mutation rates in Ashkenazi Jewish families and families of European descent, but they have been validated in black and Hispanic populations.3 BRCAPRO, originally developed as part of the DUKE Specialized Program of Research Excellence in Breast Cancer from 1995 to 1999, is a statistical model with associated software that is used to estimate the probability that a patient carries a mutation of BRCA1 or BRCA2 based on a personal or family history of breast and ovarian cancers. The BRCAPRO model identified approximately 50% of mutation-negative families, but failed to screen 10% of patients with mutation carriers.3
Breast Cancer Prevention
Since breast cancer treatment is not 100% effective, is expensive, and is associated with many adverse effects (AEs) from drug toxicities, prevention is naturally preferable. Women can reduce their risk of breast cancer by consuming a healthful diet, avoiding alcohol, controlling body weight, and exercising. The U.S. Preventive Services Task Force (USPSTF) has recommended chemoprevention—the use of medication to reduce cancer risk—for high-risk women since 2013.5 A woman is considered to be at high risk if she has one or more factors that, when combined, greatly increase breast cancer risk beyond the recommended cutoff risks. These risk factors include the following3,6:
• A personal history of invasive breast cancer or DCIS, LCIS, or atypical hyperplasia
• A strong family history of breast cancer (e.g., mother and/or sisters diagnosed with breast cancer at age 45 years or younger)
• A BRCA1 or BRCA2 gene mutation or a first-degree relative with a BRCA mutation
• Li-Fraumeni syndrome, Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome, a first-degree relative with one of these syndromes, or a family with a known p53 or PTEN gene mutation
• Radiation to the chest area before age 30 years
A clinician may use a breast cancer risk-assessment model to evaluate a patient’s breast cancer risk. If the estimated risk is high, the physician should discuss with the patient the benefits versus AE risk of breast cancer chemoprevention. If the benefit of chemotherapy is greater than the assumed risks, it is recommended that the high-risk patient take medications for breast cancer prevention.
According to the USPSTF, tamoxifen and raloxifene should be used in women with an estimated 5-year breast cancer risk of 3% or greater on the Freeman risk-assessment model. Alternatively, the American Society of Clinical Oncology (ASCO) recommends a risk cutoff of 1.67% or greater as determined by the Breast Cancer Prevention Trial. However, at the ASCO cutoff, many women will not get a net benefit from using medication for breast cancer prevention.5
TABLE 2 lists the medications that are employed for invasive breast cancer prevention. Tamoxifen (Soltamox), which was approved in 1998, has been used to treat millions of women with hormone receptor–positive breast cancer. It also is used for breast cancer prevention in high-risk women. Tamoxifen is the first choice for premenopausal women and a good choice for postmenopausal women who cannot take an aromatase inhibitor (AI).7 Tamoxifen selectively blocks or activates estrogen’s action on specific breast cells while activating estrogen’s actions in bone and liver cells. Therefore, tamoxifen helps stop bone loss after menopause and also decreases cholesterol levels. To prevent invasive breast cancer in high-risk patients, the dosage is 20 mg orally daily for 5 years. In trials, tamoxifen decreased the risk of breast cancer from 30% to 50% in premenopausal women and reduced the risk of breast cancer recurrence by 40% to 50% in postmenopausal women.5,6,8 It also decreased by 50% the risk of new cancer developing in the other breast. The most common AEs of tamoxifen are hot flashes and night sweats, nausea, fatigue, mood swings, depression, headache, hair thinning, constipation, dry skin, and loss of libido. A 2008 British study concluded that patients have complied with tamoxifen treatment because of its efficacy in preventing breast cancer recurrence. Compared with placebo, tamoxifen increased the risk of endometrial cancers (in patients with a uterus), benign gynecologic conditions, venous thromboembolic events, and uterine bleeding.5,6,8-10

Raloxifene (Evista), which was approved in 1997 to treat osteoporosis in postmenopausal women, also reduces the risk of invasive breast cancer in women with osteoporosis. Generic versions of Evista have been approved by the FDA since 2014 and are being made by various manufacturers. The dosage for prevention of breast cancer in women is 60 mg orally daily for 5 years. The MORE trial concluded that raloxifene decreased the risk of invasive breast cancer by 76%.7 The most common AEs of raloxifene are vasomotor symptoms, leg cramps, musculoskeletal problems, and weight gain. Raloxifene did not increase the risk of endometrial cancer or uterine bleeding, coronary heart disease events or stroke, cataracts, or cataract surgery. However, the USPSTF found that raloxifene increases the risk of venous thromboembolic events by 0.4% to 0.7%.6,7,10
AIs (anastrozole, letrozole, and exemestane) block activity of the enzyme aromatase, which the body uses to make estrogen in the ovaries and other tissues. AIs are used primarily to prevent invasive breast cancer in postmenopausal women. Anastrozole (Arimidex), when used for 5 years, reduced the risk of breast cancer approximately 50% in high-risk postmenopausal women, with no increase in endometrial cancer, bleeding, blood clots, or thromboembolic events.11 A study of exemestane found that this agent blocked a small amount of estrogen production in menopausal women and reduced the risk of invasive breast cancers by about 65% in healthy postmenopausal women.11 Common AEs of AIs are joint pain and stiffness and bone loss leading to osteoporosis or broken bones.10,12
Cost Savings From Using Generic Versions of Breast Cancer Medications
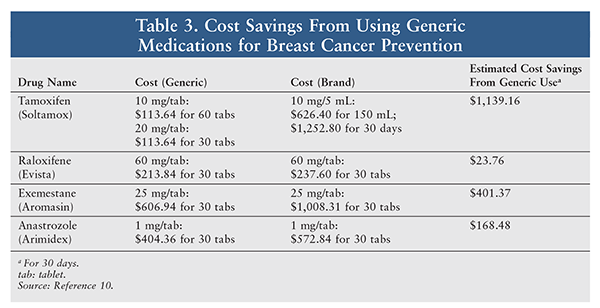
The use of medications for breast cancer prevention in high-risk women is low not only because of fears about AEs, but also because of the high cost.13 Studies have indicated that AEs and cost are responsible for noncompliance or medication cessation in 30% to 50% of patients.14 Drug cost has been identified as a modifiable factor that may improve patient compliance. Out-of-pocket drug cost is likely a major factor in patient adherence to treatment; when the monthly copayment cost goes up, adherence goes down.15 As a result, reducing the patient’s out-of-pocket medication cost through generic substitution increases adherence to breast cancer prevention.16 Since the approval of less-expensive generic versions of breast cancer medications, prevention and treatment adherence for AIs have increased by 5% to 11%. The generic version of anastrozole was approved in July 2010, followed by generic approvals for exemestane and letrozole in April 2011. Since then, the median quarterly out-of-pocket cost of anastrozole decreased from $183 in 2009 to $15 in 2011 for women without a low-income subsidy. As a result, adherence has increased from 46.3% to 52.8% for anastrozole and 57% for generic letrozole and exemestane.16 The costs of brand-name versus generic versions of drugs for breast cancer prevention are compared in TABLE 3. As TABLE 3 clearly denotes, there is a significant cost saving when generic versions of breast cancer medications are used. It is not surprising that women at high risk for breast cancer more often choose and adhere to generic versions compared with brand names.
Barriers to the Use of Generic Versions of Breast Cancer Medications
According to the FDA, generic drugs are an important option that allows greater access to healthcare for all Americans because of their cost savings. Generic medications are the same as the brand-name drugs in terms of dosage forms, safety, strength, route, quality, performance characteristics, and intended use. However, there is a common misconception that the quality of brand-name medications is better than that of generics. It is believed that efficacy of generics is inferior to that of brand-name medications. As a result, for narrow-therapeutic-index drugs, clinicians typically do not substitute generics for brand-name medications if the patient has been using the brand-name drug for a while.17 However, because of the cost and the insurance preauthorization requirement for brand-name medications, patients tend to discontinue or not comply with breast cancer prevention.13,18 Differences in pill color and shape between generic and brand-name medications also adversely impact the patient adherence (from initiation to discontinuation of therapy) to prevention or treatment.19 Finally, if generic costs potentially increase over time to approach or exceed the price of the brand-name medication, the impact of generic cost saving no longer exists.20
The Pharmacist’s Role
To enhance breast cancer prevention rates in American women, pharmacists should provide high-risk patients with information about generic medications used for breast cancer prevention, including cost, efficacy, AEs, and drug interactions. The patient should know the significant cost savings of using generics instead of brand-name medications for breast cancer prevention. Pharmacists play an important role in educating women about the efficacy of generic medications, AEs, and drug interactions. According to the FDA, “Health care professionals and consumers can be assured that FDA approved generic drug products have met the same rigid standards as the innovator drug. All generic drugs approved by FDA have the same high quality, strength, purity and stability as brand-name drugs. And, the generic manufacturing, packaging, and testing sites must pass the same quality standards as those of brand name drugs.”21 As a result, the efficacy of generic medications is equal to that of brand-name drugs.22
An FDA evaluation of 2,070 human studies from 1996 to 2007 involving absorption of brand-name drugs and clinically bioequivalent generics into the body showed that absorption did not differ significantly between brand-name drugs and their generic versions.21 It is also documented that treatment with generic drugs or switching from brand to generic formulations is not related to significant clinical failures or the development of adverse drug reactions.21 Pharmacists should explain to high-risk patients that pill color and shape do not impact the efficacy of the generic drug. It has been shown that patient education improves patients’ acceptance of generic medications in general and increases adherence to breast cancer prevention through the use of generic versions of breast cancer medications.23 Consequently, pharmacists can contribute significantly to increasing breast cancer prevention and survival rates in American women.
REFERENCES
1. American Cancer Society. What are the key statistics about breast cancer? www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key statistics. Accessed January 3, 2016.
2. Nguyen N. Contribution to breast cancer prevention for women: a challenge for pharmacists. US Pharm. 2014;39(9):35-39.
3. Stopeck AT. Breast cancer risk factors. Medscape. http://emedicine.medscape.com/article/1945957-overview. Accessed January 3, 2016.
4. Estimating breast cancer risk. Susan G. Komen. ww5.komen.org/BreastCancer/GailAssessmentModel.html. Accessed January 23, 2016.
5. Moyer VA; U.S. Preventive Services Task Force. Medications for risk reduction of primary breast cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:698-708.
6. Estimating breast cancer risk: questions and answers. www.medicinenet.com/estimating_breast_cancer_risk/article.htm. Accessed January 23, 2016.
7. Thomsen A, Kolesar JM. Chemoprevention of breast cancer. Am J Health Syst Pharm. 2008;65:2221-2228.
8. Tamoxifen for breast cancer treatment, prevention. WebMD. www.webmd.com/breast-cancer/tamoxifen-for-breast-cancer-treatment-and-prevention. Accessed January 3, 2016.
9. Radmacher MD, Simon R. Estimation of tamoxifen’s efficacy for preventing the formation and growth of breast tumors. J Natl Cancer Inst. 2000;92:48-53.
10. Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc. www.online.lexi.com. Accessed November 5, 2016.
11. Johnson K. Anastrozole halves breast cancers in prevention study. Medscape. www.medscape.com/viewarticle/817775. Accessed January 3, 2016.
12. Miller G. Exemestane well-tolerated for breast cancer prevention. Medscape. www.medscape.com/viewarticle/823789. Accessed January 3, 2016.
13. Chustecka Z. Tamoxifen not being used much for prevention of breast cancer. Medscape. www.medscape.com/viewarticle/716583. Accessed May 26, 2014.
14. Lowry F. Cheaper generic AIs boost treatment adherence. Medscape. www.medscape.com/viewarticle/850150. Accessed December 17, 2015.
15. Osterweil N. Surprise! breast cancer patients stay on affordable AIs. Medscape. www.medscape.com/viewarticle/817791. Accessed December 17, 2015.
16. Sanchez CK, Farrell N, Lapp E. Generic drugs, cost, and medication adherence. US Pharm. 2015;40(6)(Generic Drug suppl):14-19.
17. Van Amburgh JA. Brand vs generic drugs: are patient outcomes affected? Medscape. www.medscape.com/viewarticle/762489. Accessed January 23, 2016.
18. Shrank WH, Cox ER, Fischer MA, et al. Patients’ perceptions of generic medications. Health Aff (Millwood). 2009;28:546-556.
19. Kesselheim AS, Misono AS, Shrank WH, et al. Variations in pill appearance of antiepileptic drugs and the risk of nonadherence. JAMA Intern Med. 2013;173:202-208.
20. Alpern JD, Stauffer WM, Kesselheim AS. High-cost generic drugs—implications for patients and policymakers. N Engl J Med. 2014;371:1859-1862.
21. FDA.gov. Understanding generic drugs. www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingGenericDrugs/default.htm. Accessed December 28, 2015.
22. Gallelli L, Palleria C, De Vuono A, et al. Safety and efficacy of generic drugs with respect to brand formulation. J Pharmacol Pharmacother. 2013;4(suppl 1):S110-S114.
23. Van Wjjk BL, Klungel OH, Heerdink ER, de Boer A. Generic substitution of antihypertensive drugs: does it affect adherence? Ann Pharmacother. 2006;40:15-20.
To comment on this article, contact rdavidson@uspharmacist.com.






