US Pharm. 2022;47(2):HS1-HS8.
ABSTRACT: Cardio-oncology is a new discipline focused on screening, monitoring, and treating cardiovascular disease (CVD) during and after cancer treatment. One of the major areas for the application of cardio-oncology principles is breast cancer (BC). In 2018, the American Heart Association published a scientific statement on the intersection of CVD and BC that examines the scope of the problem; common risk factors associated with comorbidities; CV effects of BC treatments; monitoring parameters for assessing for CV toxicity in the BC patient; oncologic strategies to mitigate cardiotoxicity associated with BC treatments; and potential cardioprotective therapies in BC. Pharmacists can play a major role in educating patients and healthcare providers about the potential CV risk associated with BC treatment and in helping manage these complications.
In 2018, the American Heart Association (AHA) published a scientific statement on the intersection of cardiovascular disease (CVD) and breast cancer (BC). The AHA document examines the scope of the problem, common risk factors associated with comorbidities, CV effects of BC therapies, monitoring parameters to assess for CV toxicity in the BC patient, oncologic strategies to mitigate cardiotoxicity associated with BC treatments, and potential cardioprotective therapies in BC.1 This article will discuss the AHA scientific statement as well as recent developments.
CVD AND CANCER
CVD and cancer share a number of risk factors (RFs), and for BC these factors include age, diet, obesity, sedentary lifestyle, and tobacco use. Cardiac dysfunction can occur following the administration of chemotherapy and radiation therapy (RT) to the chest. CVD and BC have a bidirectional relationship in that the presence of antecedent CVD can affect the choice and dosage of chemotherapy and the treatment of BC can exacerbate or precipitate underlying heart disease. Both of these factors can ultimately influence patient survivorship.1
Despite this interrelationship, a recent survey revealed a significant knowledge gap among healthcare providers concerning prevention and treatment strategies for cancer treatment–related cardiac disease (CTRCD; involves chemotherapy and RT) and chemotherapy-related cardiac dysfunction (CRCD; refer to TABLE 1).2
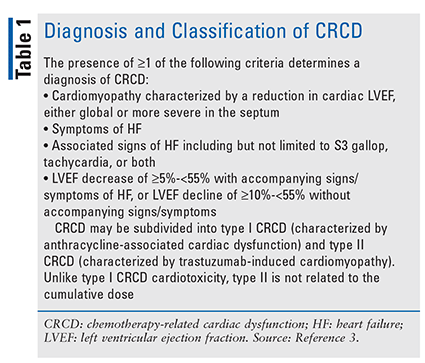
These concerns can be addressed through cardio-oncology, a new discipline focused on screening, monitoring, and treating heart disease patients during and after cancer treatment.4 One of the major areas for the application of cardio-oncology principles is BC.
SCOPE OF THE INTERSECTION OF BC AND CVD
Both CVD and BC incidence increase with age. According to the CDC, 254,744 new cases of BC were diagnosed in women in the United States in 2018.5 A woman’s risk of developing BC in her lifetime is 2.4% at age 50 years (1 in 42), 3.54% at age 60 years (1 in 28), and 4.08% by age 70 years (1 in 24).6 Heart disease is the leading cause of death among U.S. women, resulting in 299,578 deaths in 2017—or about one in every five deaths in women.7 About 68% of women aged 60 to 79 years have CVD, and the rate increases to 85% in those aged 80 years and older.8
RISK FACTORS ASSOCIATED WITH CVD AND BC
A baseline CV evaluation prior to BC treatment is essential in order to identify high-risk patients. Common RFs shared by CVD and BC include poor dietary patterns, high dietary fat, increased alcohol and meat consumption, physical inactivity, excessive body weight, tobacco use, increased age, use of postmenopausal hormone replacement therapy, and genetics.1 The risk is heightened in BC patients with preexisting CV comorbidities such as hypertension, angina, heart failure (HF), arrhythmias, atherosclerosis, coronary bypass surgery, stroke/thromboembolic events, and diabetes.4
CV EFFECTS OF BC TREATMENTS
Chemotherapeutic agents, ErbB2/HER2-targeted therapy, endocrine therapy, cyclin-dependent kinase (CDK) 4/6 inhibitors, and RT have been associated with cardiotoxicity.1
Chemotherapeutic Agents
Among the chemotherapeutic drug classes that are associated with cardiotoxicity are the anthracyclines (e.g., doxorubicin, epirubicin), the alkylating agents (e.g., cisplatin, cyclo-phosphamide), the taxanes (e.g., paclitaxel), and the antimetabolites (e.g., 5-fluorouracil, capecitabine).1
Anthracyclines: Anthracyclines commonly used in the management of BC include doxorubicin and epirubicin. These agents induce cardiotoxicity by interacting with DNA, causing the intercalation and inhibition of macromolecular and mitochondrial biosynthesis. They also act by inhibiting topoisomerase II-beta within the myocytes. These actions result in the disruption of myocyte function and death.9 Anthracyclines may damage myocytes by generating reactive oxygen species (ROS) and by forming complexes with intracellular iron, which also leads to the formation of free radicals. Anthracyclines may impair iron-metabolism pathways, resulting in iron accumulation in the cardiomyocytes. Higher iron stores are associated with an increased risk of cardiotoxicity.3,9 RFs for anthracycline-induced cardiotoxicity are listed in TABLE 2.
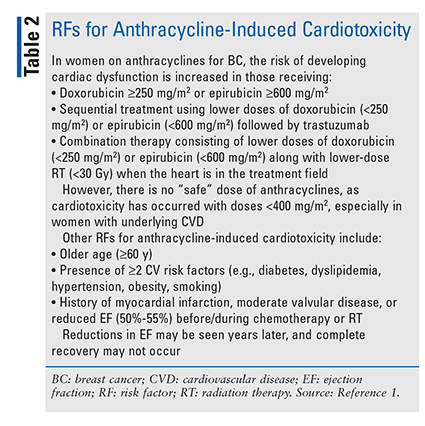
An early manifestation of anthracycline-induced cardiotoxicity may be pericarditis-myocarditis syndrome; subsequently, cardiac adverse effects (AEs) may present as HF.1 The cardionecrotic effects of anthracyclines are evidenced by rising troponin levels.1 However, anthracycline-treated BC patients often remain asymptomatic despite decreases in left ventricular ejection fraction (LVEF).10 Anthracycline-induced cardiotoxicity includes ventricular tachycardia, atrial fibrillation (occurring during or following treatment), ventricular fibrillation, and prolonged corrected QT interval (QTc).1
Epirubicin has not been shown to be less cardiosparing than doxorubicin.11 RFs for epirubicin cardiotoxicity include the number of anthracycline cycles, modestly elevated blood pressure (i.e., 140/90 mmHg or greater), increased body-surface area, and trastuzumab use.12
The European Society of Medical Oncology (ESMO) reported LVEF decreases of 7%, 16%, and 20% at doxorubicin dosages of 200 mg/m2, 400 mg/m2, and 500 mg/m2, respectively. About 12% of patients with normal LVEF at the time of completing anthracycline treatment develop LVEF decreases in the future. Subclinical cardiac damage may become irreversible once LVEF develops.13
Alkylating Agents (AAs): The AAs cisplatin and cyclophosphamide are commonly used in BC management. The mechanism by which AAs appear to cause cardiotoxicity involves interstitial hemorrhage, edema, fibrosis, disruption and aggregation of mitochondrial cristae, and necrosis.1,14 Like anthracyclines, AAs also produce cardiotoxicity secondary to mitochondrial dysfunction.15 Increased ROS generation and reduced aldehyde dehydrogenase activity secondary to cyclophosphamide’s metabolites (4-hydroxy-cyclophosphamide and acrolein) are thought to be linked to cyclophosphamide-induced cardiotoxicity.16,17 Cardiotoxicity (including myocarditis and pericarditis) is uncommon with cyclophosphamide, but the risk increases with prior use of anthracyclines or mediastinal RT and high doses of cyclophosphamide.1,17 Cyclophosphamide-induced cardiotoxicity usually occurs early (i.e., within the first 10 days of therapy).1
Cisplatin has been associated with cardiac AEs including chest pain (occurring at a rate as high as 40% and believed to be due to endothelial dysfunction and altered vasoreactivity); coronary thrombosis (even in patients without underlying atherosclerosis); arrhythmias; acute ischemic events (including ischemia of limbs and myocardial infarction [MI] secondary to abnormal vasoreactivity); and stroke (may be caused by endothelial cell death and production of procoagulant microparticles). Uncommonly, cisplatin has been associated with acute thrombotic occlusions of the aorta and peripheral arteries. Cisplatin levels may be detectable for years following therapy, leaving patients at continued risk for chest-pain episodes and acute ischemic events.18
Taxanes: The taxanes that are most often used in BC management are paclitaxel and docetaxel. Taxanes act by interfering with microtubule processes and disrupting mitosis, which results in apoptosis. Paclitaxel has been associated with a bradycardia incidence of 29% as well as with asymptomatic left bundle-branch block and nonsustained ventricular tachycardia. Taxanes are often employed concomitantly or sequentially with anthracyclines, but this regimen does not appear to directly increase HF risk.1
Antimetabolites (Fluoropyrimidines): The antimetabolites, which include 5-fluorouracil and capecitabine, act by disrupting DNA and RNA synthesis. Capecitabine is a prodrug of 5-fluorouracil. This class of oncologic agents is often used in combination with anthracyclines and AAs.1 These drugs exert their cardiotoxic effect by inducing thrombosis or coronary arterial vasospasm and by direct cytotoxicity.1,19 The most common cardiac AE is chest pain, but MI, HF, and arrhythmias have also been reported.1 ST-T wave changes account for 69% of cardiac events, and angina, arrhythmias, MI, abnormal cardiac enzymes, pulmonary embolism, cardiac arrest/pericarditis, and cardiogenic shock have been reported in 45%, 23%, 22%, 12%, 5%, 1.4%, and 1%, respectively. However, it is believed that the cardiotoxic effects of 5-fluorouracil are grossly underestimated.20 In addition to these AEs, cardiomyopathy, sinoatrial and atrioventricular nodal dysfunction, takotsubo cardiomyopathy (i.e., stress-induced cardiopathy), and torsades de pointes secondary to QT prolongation have occurred.21 The incidence of cardiotoxicity from 5-fluorouracil appears to be dose-related.1
Ischemia is more likely in patients with underlying coronary artery disease (CAD) than in those without CAD (4.5% vs. 1.1%, respectively). Coronary vasospasm can occur at any time from 5-fluorouracil infusion through 5 days after administration.1 With capecitabine, coronary vasospasm may occur later—i.e., after 4 days of treatment—owing to the need for activation of the prodrug.19 In addition to prior history of CAD, other RFs for 5-fluorouracil cardiotoxicity include previous mediastinal radiation and concurrent combination antineoplastic therapy. Coronary artery spasm can induce ventricular arrhythmias, but atrial fibrillation, atrial/ventricular ectopy, and QT prolongation have also been reported.1
A recent review examined the manifestations, mechanisms, and management of fluoropyrimidine-induced cardiotoxicity. The investigators of this study developed a proposed algorithm that outlined management strategies for suspected fluoropyrimidine cardiotoxicity.19
ErbB2/HER2-Targeted Therapies
ErbB2/HER2-targeted therapies that are associated with cardiac AEs include the monoclonal antibodies trastuzumab and pertuzumab as well as the small-molecule tyrosine kinase inhibitor lapatinib.1
Monoclonal Antibodies: Trastuzumab and pertuzumab are monoclonal antibodies that target the ErbB2/HER2 receptor. ErbB2/HER2-targeted therapies are thought to induce cardiotoxicity—in particular, left ventricular (LV) dysfunction—by binding to the extracellular domain of the ErbB2/HER2 receptor, which leads to reduced ErbB2 signaling in cardiomyocytes.1 The LV dysfunction and HF associated with trastuzumab are generally reversible once therapy is discontinued.1 TABLE 3 lists RFs for trastuzumab-induced cardiotoxicity.1,22
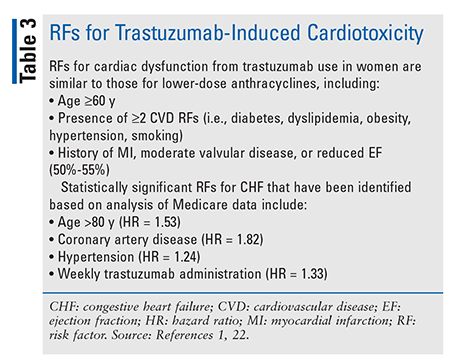
The incidence of cardiac events associated with trastuzumab varies based on RFs but ranges from 0% to 3.9%.1 Length of therapy is a major factor in the development of grade 3/4 AEs, and reductions in LVEF were reported more frequently when trastuzumab was administered for 2 years versus 1 year (20.4% vs. 16.3% for grade 3/4 AE; 7.2% vs. 4.1% for decreases in LVEF).23
ESMO reported a cumulative percentage of LVEF reduction of 10% at 3 months, 19% at 6 months, and 25% at 12 months of therapy in patients on trastuzumab treatment who had previously received anthracycline therapy. Approximately 10% of trastuzumab-treated patients, regardless of whether they received anthracycline therapy, will develop reduced LVEF after 1 year of treatment.13
Trastuzumab carries a boxed warning for LV cardiac dysfunction, arrhythmias, hypertension, disabling cardiac failure, cardiomyopathy, and cardiac death, as well as an asymptomatic decline in LVEF. Compared with patients not receiving trastuzumab, those who receive it as a single agent or in combination therapy have a fourfold to sixfold increase in the incidence of symptomatic myocardial dysfunction. The highest absolute incidence occurs when trastuzumab is administered with an anthracycline. Among BC patients who received doxorubicin and cyclophosphamide prior to trastuzumab, the incidence of New York Heart Association III-IV HF was 19%.24 A recent review of anthracycline- and trastuzumab-induced cardiotoxicity calls for the early identification and prompt treatment of subclinical cardiotoxicity secondary to these agents.25
Trastuzumab should be withheld for a 16% or greater absolute decrease in LVEF from pretreatment values or an LVEF value below institutional limits of normal and a 10% or greater absolute decrease in LVEF from pretreatment values. Patients receiving trastuzumab should have a baseline LVEF measurement immediately prior to initiation of therapy; LVEF measurements every 3 months during and upon completion of therapy; subsequent LVEF measurements at 4-week intervals if trastuzumab is withheld for significant LV cardiac dysfunction; and LVEF measurements every 6 months for at least 2 years following completion of therapy.24
Small-Molecule Tyrosine Kinase Inhibitors: Lapatinib is a kinase inhibitor of the intracellular tyrosine kinase domains of both epidermal growth factor receptor (ErbB1) and ErbB2/HER2 receptors. It is indicated for women with hormone receptor–positive metastatic BC that overexpresses the ErbB2/HER2 receptor and is given in conjunction with letrozole or capecitabine.26 Lapatinib carries a warning regarding decreased LVEF. In clinical trials, the majority (>57%) of LVEF decreases occurred within the first 12 weeks of treatment. Caution is advised when the drug is administered to patients who have conditions that could impair LV function.26
Endocrine Therapy
Endocrine therapies for BC that have been associated with cardiac AEs are tamoxifen and the aromatase inhibitors (AIs; anastrozole, letrozole, and exemestane).
Tamoxifen: Tamoxifen is a selective estrogen receptor modulator that interferes with estrogen binding at the estrogen receptor (ER), resulting in the alteration of gene expression downstream from the ER. Tamoxifen is associated with a 10% to 15% reduction in total serum cholesterol and a 15% to 22% reduction in LDL cholesterol; it has no effect on HDL. An increase in triglycerides has been observed in Asian populations. Several older major clinical trials failed to demonstrate cardioprotective benefits from the use of tamoxifen.27,28 A recent systematic review and meta-analysis comparing the cardiotoxicity of tamoxifen versus AIs found that AIs increased the risk of CV AEs by 19% compared with tamoxifen and that tamoxifen reduced CV risk by 33% compared with placebo or no treatment.29
Tamoxifen is known to be thrombogenic secondary to its agonistic effect at the ER. This agent carries a boxed warning about the risk of stroke and pulmonary emboli and can also have long-term sequelae such as pulmonary hypertension.30 The incidence of thrombosis is about 2.8%.1
A review of the European database of suspected adverse drug events observed that tamoxifen was associated with more reports of drug-induced prolonged QT interval, torsades de pointes, and ventricular arrhythmias compared with AIs.31
AIs: AIs, which include anastrozole, letrozole, and exemestane, block the peripheral conversion of androstenedione to estradiol by inhibiting the aromatase enzyme, which results in estrogen depletion.1 AIs produce CV AEs by reducing endothelial function.32 The effect of AIs on the lipid profile remains unclear, with some studies showing a higher incidence of hypercholesterolemia with anastrozole and letrozole and others not demonstrating a significant difference.33-35 Letrozole carries a warning about the risk of hypercholesterolemia.36 Longer duration of use and order of administration (e.g., tamoxifen first followed by sequential AI, or AI used from the beginning) may be associated with an increased risk of cardiotoxicity (e.g., 26-fold increase for this example).37,38
Other studies have produced mixed results as to whether the risks of MI, myocardial ischemia, stroke, dysrhythmias, valvular dysfunction, pericarditis, HF, and cardiomyopathy are increased for AIs compared with tamoxifen.1 Left-sided chest radiation for BC in conjunction with AI therapy leads to adverse changes in right ventricular systolic function and LV diastolic function.39
Anastrozole carries a warning that an increased incidence of ischemic CV events (17%) has been found to occur in women with preexisting ischemic heart disease compared with tamoxifen (10%). Clinicians should consider the risks and benefits of using this drug.40 Exemestane’s labeling does not carry any CV AE warnings.41
CDK 4/6 Inhibitors
Palociclib, ribociclib, and abemaciclib—small-molecule CDK inhibitors that are selective for CDK 4 and CDK 6—are indicated for metastatic BC. CDKs are regulators of the cell cycle, and in cancer, CDKs are upregulated and promote tumor growth by inhibiting tumor suppression and apoptosis of malignant cells. CDK 4/6 inhibitors block retinoblastoma protein phosphorylation and prevent progression through the cell cycle from G1 to the S cell cycle phase.1 TABLE 4 contains product-labeling warnings regarding CDK 4/6 inhibitors and CTRCD.42-44
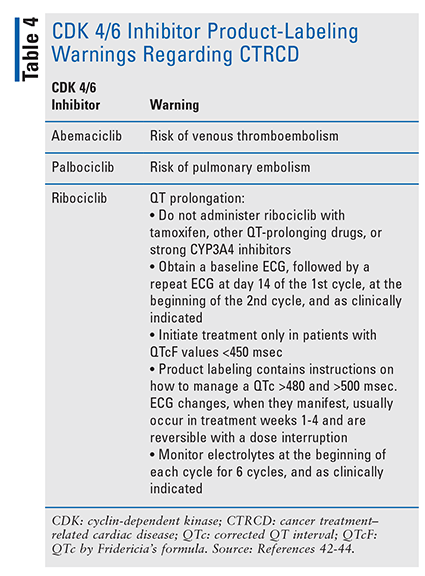
Radiation Therapy
Radiation-induced cardiotoxicity is thought to be due to the induction of myocyte ischemia/injury and fibrosis secondary to microvascular dysfunction, inflammation, and oxidative stress and the formation of foam cells that initiate atherosclerosis.1
MONITORING PARAMETERS FOR CV TOXICITY IN BC PATIENTS
Early Identification of CVD Risk
It is important to identify CVD risk early in the BC patient. Echocardiography, multigated acquisition scan, and cardiac MRI have been used to define risk, although consensus is lacking on what constitutes a clinically significant reduction in cardiac function. Newer technologies such as myocardial strain imaging (i.e., a principle for quantifying LV function that represents percent change in myocardial length from relaxed to contractile state) for early risk identification and the use of biomarkers for chemotherapy-induced cardiac disease can aid in early diagnosis.1,45-49 Biomarkers include troponin 1, high-sensitivity troponin 1, myeloperoxidases, placental growth factor, and growth differentiation factor 15.1,50-54
ESMO recommends that patients who are receiving a cardiotoxic chemotherapeutic agent get a baseline ECG, including QTc. If the chemotherapeutic agent to be administered is associated with the development of HF or LV dilation, baseline evaluation of LVEF and diastolic function should be performed. The guidelines suggest that baseline measurement of cardiac biomarkers be considered for high-risk patients (i.e., with preexisting significant CVD) and for patients who are receiving high doses of cardiotoxic chemotherapy such as anthracycline.13
MITIGATION STRATEGIES FOR BC TREATMENT–ASSOCIATED CARDIOTOXICITY
Because the development of CTRCD can adversely affect treatment completion and survivorship, the search is on for interventions that can help mitigate the cardiotoxic effects of BC treatments. Among these interventions are the use of dexrazoxane, prolongation of administration of doxorubicin when it is part of a treatment regimen, and modification of radiation techniques.1
Mitigation Strategies for CTRCD
Dexrazoxane: Dexrazoxane, an intracellular chelating agent that interferes with iron-mediated free-radical generation, alters the conformation of topoisomerase II-beta to interfere with anthracycline binding. Dexrazoxane therapy in patients receiving doxorubicin or epirubicin has been associated with beneficial effects on LVEF or HF. Although it has been shown to reduce cardiac events by 65% and HF by 79% to 82%, dexrazoxane has not affected progression-free survival, overall survival, or response rates. The development of secondary malignancies and reduced tumor response is of concern with dexrazoxane use.1
Dexrazoxane is contraindicated with non-anthracycline regimens. It is indicated only for patients who have received a cumulative doxorubicin dose of 300 mg/m2 and are continuing with doxorubicin therapy. It should not be used upon initiation of chemotherapy, as it may interfere with the antitumor activity of the chemotherapy regimen (e.g., fluorouracil, doxorubicin, cyclophosphamide), leading to lower response rates (48% vs. 63%) and shorter time to progression versus patients given placebo.55
Doxorubicin Administration: Administration techniques for doxorubicin, including rate of infusion, may affect CTRCD. Continuous infusion dosing (48-96 hours), as opposed to bolus dosing, may be cardioprotective. Bolus dosing of doxorubicin or epirubicin may increase the risk of CV toxicity more than fourfold.1,56 A Cochrane database review found that increasing infusion to 6 hours or more was associated with a 73% reduction in clinical HF.57 A drawback of continuous infusion of doxorubicin is that because of its prolonged length, hospitalization is required.1
The use of liposomal (pegylated or nonpegylated) doxorubicin allows for the delivery of a larger cumulative dose while limiting toxicity. It restricts the chemotherapeutic agent’s distribution in cardiac tissue by prohibiting passage through narrow capillary junctions, and it can reduce the incidence of CV AEs by 82%.1,58 Liposomal doxorubicin is off-label for metastatic BC.59
The American Society of Clinical Oncology recommends that clinicians incorporate a number of strategies, such as dexrazoxane, continuous infusion of doxorubicin, or liposomal formulations of doxorubicin, for prevention of cardiotoxicity in patients planning to receive high-dose anthracyclines (e.g., doxorubicin 250 mg/m2 or more, epirubicin 600 mg/m2 or more).60
Radiation Techniques: Techniques that have been used to limit radiation dose, dose per fraction, and volume of heart exposed include proton therapy, deep-inspiration breath holding, respiratory gating, lateral decubitus positioning, and modern three-dimensional planning.1
POTENTIAL CARDIOPROTECTIVE THERAPIES IN BC
Despite their benefits, mitigation strategies have limitations too. Several classes of medications, including beta-blockers (BBs), renin-angiotensin-aldosterone system (RAAS) antagonists, and statins, are being investigated for their potential cardioprotective effects.1
Beta-Blockers
The data on BBs are mixed but point to a cardioprotective effect on LVEF that persists for at least 6 months when the agent is initiated prior to anthracycline therapy. BBs may also have advantages for patients on trastuzumab therapy. Carvedilol, nebivolol, and candesartan are BBs that have demonstrated beneficial effects on CV function in BC patients.1
A recent meta-analysis that evaluated the cardioprotective effect of BBs in preventing anthracycline-induced cardiotoxicity found that BB use was associated with significantly increased LVEF compared with placebo.61 Although analysis revealed favorable trends indicating beneficial effects of BBs on diastolic function, global longitudinal strain, and LV end-diastolic diameter (LVEDD), only the last parameter reached statistical significance, and LVEDD may be a risk factor for sudden death.62
In the largest clinical trial (N = 200) exploring BB use for anthracycline-induced cardiotoxicity, carvedilol (maximum dosage 25 mg every 8 hours) did not affect early LVEF reduction; however, it decreased troponin I levels and diastolic dysfunction, indicating a protective effect against myocardial injury.63
RAAS Blockade
A recent meta-analysis of controlled trials examining the use of ACE inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) in the prevention of anthrcycline-induced cardiotoxicity demonstrated that patients who received RAAS blockade had better LVEF preservation without evidence of hypotension.64 Additional evidence indicates that ACEIs/ARBs may lessen CTRCD following the administration of anthracyclines and/or trastuzumab.65
ACEIs may be combined with dexrazoxane to help prevent cardiotoxicity in metastatic BC patients receiving epirubicin. ACEIs are also beneficial when combined with doxorubicin to prevent LVEF declines.66 Spironolactone 25 mg/day administered concomitantly with an anthracycline demonstrated protective effects on systolic and diastolic myocardial function.67
BBs/ACEIs/ARBs for Cardiotoxicity: Early treatment of anthracycline- or trastuzumab-induced cardio-myopathy with a combination of a BB and a RAAS-blocking agent can lead to LVEF improvement; however, the patient may not always return to baseline LV function. Only about three-quarters of trastuzumab-treated patients return to baseline LV systolic function, with a median time of recovery of 7 months. The use of RAAS inhibitors and BBs and lower anthracycline doses were associated with LV functional reversibility.68
ACEI/BB combination therapy has been found useful for preventing trastuzumab- or anthracycline-induced cardiotoxicity in older women (age 66 years and older), as it reduced cardiotoxicity by 23% and all-cause mortality by 21%. Initiating cardioprotective therapy less than 6 months following treatment with anthracycline/trastuzumab and maintaining it for less than 6 months was most beneficial.69
A systematic review and meta-analysis revealed that patients on higher cumulative anthracycline doses benefited most from BB and RAAS cardioprotection.70 A different meta-analysis, which examined the pooled effect estimate of the potential benefits and harms of BB/ACEI/ARB combination therapy in BC patients receiving anthracycline with or without trastuzumab, found a significantly lower difference in mean change of LVEF immediately following chemotherapy.71 This cardioprotective benefit persisted at 12 months, and the incidence of HF was reduced by 88%. However, combination therapy did not affect end-systolic or end-diastolic volume.71
The Canadian Cardiovascular Society’s guidelines on the evaluation and management of CV complications during cancer therapy recommend that ACEIs, ARBs, and BBs be used in patients with an LVEF under 40%. They may also be considered in patients who have had an asymptomatic decline in LVEF (e.g., >10% decrease in LVEF from baseline) or whose LVEF drops less than 53% while on chemotherapy.72 The AHA Scientific Statement on BC and CVD recommends treating anthracycline- or trastuzumab-induced cardiomyopathy according to the American College of Cardiology/American Heart Association HF guidelines.73,74
Statins
In women with BC receiving trastuzumab with or without an anthracycline, statin use was associated with a 68% lower risk of cardiotoxicity compared with statin non-use.75 Previously, similar results were found in a larger study in women receiving anthracycline chemotherapy for BC.76 Uninterrupted statin use (follow-up period: 2.55 years) was associated with a 70% decrease for new-onset HF.76
Exercise
Researchers have investigated the role of exercise and physical activity in reducing the risk of chemotherapy-induced CVD; however, these studies have yielded mixed results.1
SURVIVORSHIP PROGRAMS
As treatment strategies for managing BC improve, the focus is shifting to addressing CV comorbidity either from preexisting CVD or from CTRCD. The identification of BC patients who are at high risk for CVD (e.g., have received >240 mg/m2 of doxorubicin or >30 Gy of radiation plus an anthracycline, or high-dose cyclophosphamide) is essential. Current HF guidelines should be followed in the management of these patients. Pharmacists can be involved in managing chronic diseases such as hypertension, HF, dyslipidemia, and diabetes in order to help mitigate the effects of CTRCD.1
PHARMACIST’S ROLE IN MANAGING BC AND CVD COMORBIDITIES
A recent survey found that only 35% of oncologists used oncology expert guidelines and none had used cardiology expert guidelines to manage CRCD. The management of hypertension and QT prolongation, two fairly common CV AEs associated with chemotherapy for BC, were inconsistently treated by oncologists.77
Pharmacists can play a major role in educating patients—especially older adults—and healthcare providers about the potential CV risk associated with BC treatment and in helping manage these complications. Additionally, pharmacists can encourage the use of screening tests for cardiotoxicity, the initiation of cardioprotective interventions, and the use of mitigation strategies should toxicity occur.
CONCLUSION
Although chemotherapeutic agents and biological agents have significantly decreased morbidity and mortality among BC patients, these agents often carry the risk of significant CV AEs. It is important for pharmacists to recognize these risks in order to minimize long-term CV sequelae in their patients with BC.
REFERENCES
1. Mehta LS, Watson KE, Barac A, et al; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30-e66.
2. Peng J, Rushton M, Johnson C, et al. An international survey of healthcare providers’ knowledge of cardiac complications of cancer treatments. Cardio-Oncology. 2019;5:12.
3. Saidi A, Alharethi R. Management of chemotherapy induced cardiomyopathy. Curr Cardiol Rev. 2011;7(4):245-249.
4. Kostakou PM, Kouris NT, Kostopoulos VS, et al. Cardio-oncology: a new and developing sector of research and therapy in the field of cardiology. Heart Fail Rev. 2019;24(1):91-100.
5. CDC. Cancer statistics at a glance. Leading cancer causes of death, all races and ethnicities, female, 2018: female breast. https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/. Accessed June 24, 2021.
6. National Cancer Institute. Breast cancer risk in American women. www.cancer.gov/types/breast/risk-fact-sheet. Accessed June 24, 2021.
7. CDC. Women and heart disease. www.cdc.gov/heartdisease/women.htm. Accessed June 24, 2021.
8. American Heart Association. Older Americans & cardiovascular diseases. www.heart.org/idc/groups/heart-public/@wcm/@sop/@smd/documents/downloadable/ucm_472923.pdf. Accessed June 24, 2021.
9. Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971-977.
10. Dent SF, Botros J, Rushton M, et al. Anthracycline-induced cardiotoxicity in patients with early-stage breast cancer: the Canadian Cancer Trials Group (CCTG) MA.21 experience. Breast Cancer Res Treat. 2020;184(3):733-741.
11. van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010(5):CD005006.
12. Kotwinski P, Smith G, Cooper J, et al; Breast cancer Early disease: Toxicity from Therapy with Epirubicin Regimens–Cardiac Assessment and Risk Evaluation (BETTER-CARE) Study Investigators. Body surface area and baseline blood pressure predict subclinical anthracycline cardiotoxicity in women treated for early breast cancer. PLoS One. 2016;11(12):e0165262.
13. Curigliano G, Lenihan D, Fradley M, et al; ESMO Guidelines Committee. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171-190.14. Kusumoto S, Kawano H, Hayashi T, et al. Cyclophosphamide-induced cardiotoxicity with a prolonged clinical course diagnosed on an endomyocardial biopsy. Intern Med. 2013;52(20):2311-2315.
15. Finsterer J, Ohnsorge P. Influence of mitochondrion-toxic agents on the cardiovascular system. Regul Toxicol Pharmacol. 2013;67(3):434-445.16. Kurauchi K, Nishikawa T, Miyahara E, et al. Role of metabolites of cyclophosphamide in cardiotoxicity. BMC Res Notes. 2017;10(1):406.
17. Nishikawa T, Miyahara E, Kurauchi K, et al. Mechanisms of fatal cardiotoxicity following high-dose cyclophosphamide therapy and a method for its prevention. PLoS One. 2015;10(6):e0131394.18. Herrmann J, Yang EH, Iliescu CA, et al. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation. 2016;133(13):1272-1289.
19. Layoun ME, Wickramasinghe CD, Peralta MV, Yang EH. Fluoropyrimidine-induced cardiotoxicity: manifestations, mechanisms, and management. Curr Oncol Rep. 2016;18(6):35.20. Steger F, Hautmann MG, Kölbl O. 5-FU-induced cardiac toxicity—an underestimated problem in radiooncology? Radiat Oncol. 2012;7:212.
21. Stewart T, Pavlakis N, Ward M. Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern Med J. 2010;40(4):303-307.22. Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31(33):4222-4228.
23. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al; Herceptin Adjuvant (HERA) Trial Study Team. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021-1028.24. Herceptin (trastuzumab) package insert. South San Francisco, CA: Genentech, Inc; February 2021.
25. Nicolazzi MA, Carnicelli A, Fuorlo M, et al. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur Rev Med Pharmacol Sci. 2018;22(7):2175-2185.26. Tykerb (lapatinib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; February 2021.
27. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451-1467.28. Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93(9):684-690.
29. Khosrow-Khavar F, Filion KB, Al-Qurashi S, et al. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2017;28(3):487-496.30. Tamoxifen citrate package insert. Parsippany, NJ: Actavis Pharma, Inc; April 2015.
31. Grouthier V, Lebrun-Vignes B, Glazer AM, et al. Increased long QT and torsade de pointes reporting on tamoxifen compared with aromatase inhibitors. Heart. 2018;104(22):1859-1863.32. Blaes A, Beckwith H, Florea N, et al. Vascular function in breast cancer survivors on aromatase inhibitors: a pilot study. Breast Cancer Res Treat. 2017;166(2):541-547.
33. Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group; Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7(8):633-643.34. Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25(5):486-492.
35. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97(17):1262-1271.36. Letrozole package insert. Las Vegas, NV: Yiling Pharmaceutical, Inc; September 2019.
37. Zhao F, Ren D, Shen G, et al. Toxicity of extended adjuvant endocrine with aromatase inhibitors in patients with postmenopausal breast cancer: a systemic review and meta-analysis. Crit Rev Oncol Hematol. 2020;156:103114.38. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299-1309.
39. Skyttä T, Tuohinen S, Virtanen V, et al. The concurrent use of aromatase inhibitors and radiotherapy induces echocardiographic changes in patients with breast cancer. Anticancer Res. 2015;35(3):1559-1566.40. Arimidex (anastrazole) package insert. Baudette, MN: ANI Pharmaceuticals, Inc; August 2019.
41. Aromasin (exemestane) package insert. New York, NY: Pfizer Inc; May 2018.42. Verzenio (abemaciclib) package insert. Indianapolis, IN: Lilly USA, LLC; March 2020.
43. Ibrance (palbociclib) package insert. New York, NY: Pfizer Inc; February 2016.44. Kisqali (ribociclib tablet) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; July 2020.
45. Amzulescu MS, De Craene M, Langet H, et al. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging. 2019;20(6):605-619.46. Smiseth OA, Torp H, Opdahl A, et al. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37(15):1196-1207.
47. Karlsen S, Dahlslett T, Grenne B, et al. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc Ultrasound. 2019;17(1):18.48. Yang H, Wright L, Negishi T, et al. Research to practice: assessment of left ventricular global longitudinal strain for surveillance of cancer chemotherapeutic-related cardiac dysfunction. JACC Cardiovasc Imaging. 2018;11(8):1196-1201.
49. Fei HW, Ali MT, Tan TC, et al. Left ventricular global longitudinal strain in HER-2 + breast cancer patients treated with anthracyclines and trastuzumab who develop cardiotoxicity is associated with subsequent recovery of left ventricular ejection fraction. Echocardiography. 2016;33(4):519-526.50. Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25(6):1102-1111.
51. Putt M, Hahn VS, Januzzi JL, et al. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem. 2015;61(9):1164-1172.52. Matsui M, Uemura S, Takeda Y, et al. Placental growth factor as a predictor of cardiovascular events in patients with CKD from the NARA-CKD study. J Am Soc Nephrol. 2015;26(11):2871-2881.
53. Chau K, Hennessy A, Makris A. Placental growth factor and pre-eclampsia. J Hum Hypertens. 2017;31:782-786.54. Mayo Clinic Laboratories. Test ID: GDF15. Growth differentiation factor 15, plasma. www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/64637. Accessed June 24, 2021.
55. Dexrazoxane package insert. Morristown, NJ: Almaject, Inc; May 2019.56. Padegimas A, Clasen S, Ky B. Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends Cardiovasc Med. 2020;30(1):22-28.
57. van Dalen EC, van der Pal HJ, Kremer LC. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2016;3(3):CD005008.58. Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337.
59. Doxorubicin (liposomal). In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2021. http://online.lexi.com/. Accessed June 24, 2021.60. Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893-911.
61. Dempsey N, Rosenthal A, Dabas N, et al. Trastuzumab-induced cardiotoxicity: a review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res Treat. 2021;188(1):21-36.62. Narayanan K, Reinier K, Teodorescu C, et al. Left ventricular diameter and risk stratification for sudden cardiac death. J Am Heart Assoc. 2014;3(5):e001193.
63. Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71(20):2281-2290.64. Lin H, Liang G, Wu Y, Chen L. Protective effects of ACEI/ARB on left ventricular function in anthracycline-induced chronic cardiotoxicity: a meta-analysis of randomized controlled trials. Cardiology. 2021;146(4):469-480.
65. Fang K, Zhang Y, Liu W, He C. Effects of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use on cancer therapy-related cardiac dysfunction: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2021;26(1):101-109.66. Ghasemi K, Vaseghi G, Mansourian M. Pharmacological interventions for preventing anthracycline-induced clinical and subclinical cardiotoxicity: a network meta-analysis of metastatic breast cancer. J Oncol Pharm Pract. 2021;27(2):414-427.
67. Akpek M, Ozdogru I, Sahin O, et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail. 2015;17(1):81-89.68. Ohtani K, Ide T, Hiasa KI, et al. Cardioprotective effect of renin-angiotensin inhibitors and beta-blockers in trastuzumab-related cardiotoxicity. Clin Res Cardiol. 2019;108(10):1128-1139.
69. Wittayanukorn S, Qian J, Westrick SC, et al. Prevention of trastuzumab and anthracycline-induced cardiotoxicity using angiotensin-converting enzyme inhibitors or beta-blockers in older adults with breast cancer. Am J Clin Oncol. 2018;41(9):909-918.70. Yun S, Vincelette ND, Abraham I. Cardioprotective role of beta-blockers and angiotensin antagonists in early-onset anthracyclines-induced cardiotoxicity in adult patients: a systematic review and meta-analysis. Postgrad Med J. 2015;91(1081):627-633.
71. Elghazawy H, Venkatesulu BP, Verma V, et al. The role of cardio-protective agents in cardio-preservation in breast cancer patients receiving anthracyclines ± trastuzumab: a meta-analysis of clinical studies. Crit Rev Oncol Hematol. 2020;153:103006.72. Virani SA, Dent S, Brezden-Masley C, et al. Canadian Cardiovascular Society guidelines for evaluation and management of cardiovascular complications of cancer therapy. Can J Cardiol. 2016;32(7):831-841.
73. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803.74. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
75. Calvillo-Argüelles O, Abdel-Qadir H, Michalowska M, et al. Cardioprotective effect of statins in patients with HER2-positive breast cancer receiving trastuzumab therapy. Can J Cardiol. 2019;35(2):153-159.76. Seicean S, Seicean A, Plana JC, et al. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60(23):2384-2390.
77. Jovenaux L, Cautela J, Resseguier N, et al. Practices in management of cancer treatment-related cardiovascular toxicity: a cardio-oncology survey. Int J Cardiol. 2017;241:387-392.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






