US Pharm. 2017;42(9):12-16.
ABSTRACT: Endometriosis is an estrogen-mediated growth of endometrial tissue outside the uterus. Patients with this gynecologic condition, for which there is no cure, experience debilitating pelvic pain during menstruation and have a greater chance of infertility. Endometriosis affects about 10% of women of childbearing age. Management of endometriosis involves the use of nonsteroidal anti-inflammatory drugs and hormonal therapies, which have been observed to reduce endometrial proliferation. Following an evaluation of patient-specific toxicities, oral contraceptives, progestins, danazol, or gonadotropin-releasing hormone agonists are used to suppress estrogen levels. Surgery is considered if a pelvic mass is detected or if the patient is planning a pregnancy.
Endometriosis, which is the estrogen-dependent growth of endometrial tissue outside the uterus, causes inflammation, pelvic pain, dysmenorrhea, painful intercourse, and infertility in approximately 10% of females of reproductive age.1 As of 2010, an estimated 176 million women of childbearing age worldwide were affected.2 Although it is considered a benign disorder, endometriosis can affect the patient’s quality of life. In addition to causing debilitating pain, endometriosis results in worse clinical outcomes in women undergoing assisted reproductive technology, adding to the negative social and psychological impact of this chronic gynecologic condition.3 Endometriosis occurs rarely in postmenopausal women.4
Pathogenesis and Clinical Presentation
Several theories have been proposed for the pathogenesis of endometriosis. It was first hypothesized by Sampson that women with prolonged menses have an increased risk because of the retrograde flow of sloughed endometrial cells into the pelvic cavity from the fallopian tubes.5 The tissue implants onto the peritoneum, resulting in the growth of lesions. Estrogen plays a role in this process by promoting cellular proliferation.6 Patients with endometriosis have higher concentrations of activated macrophages, interleukins, and tumor necrosis factor, as well as repressed natural killer cell formation, further promoting lesion growth in the area and preventing the elimination of endometrial debris.7-9 The development of endometriosis is characterized by an increase in cyclooxygenase-2 (COX-2), which results in prostaglandin excess and inflammation and in enhanced aromatase activity that raises estrogen levels. Progesterone resistance also occurs.10 A genetic component should not be excluded because endometriosis has an estimated heritability of about 50%.11
A patient with endometriosis experiences consistent premenstrual pelvic and lower back pain that may be alleviated following menstruation. Endometriosis-related infertility is thought to be caused by the aforementioned development of lesions accompanied by pelvic distortion or by the presence of an ovarian cyst known as an endometrioma, or “chocolate cyst.” Endometriomas adhere to surrounding structures, such as the fallopian tubes, and are suggested to increase the risk of ovarian cancer.12
Diagnosis and Treatment
No individual markers have been identified that are specific to the diagnosis of endometriosis, and there are no detectable laboratory abnormalities. An experienced physician may be able to obtain a diagnosis during the patient history or palpation of the pelvic area. The most useful diagnostic technique remains laparoscopy, with suspicious lesions biopsied for histologic confirmation.13 There is no cure for endometriosis. Clinical management may be achieved through surgery or by using drugs that modify the abovementioned mechanisms of pathogenesis, including medication for the relief of pain.
Pharmacologic Management
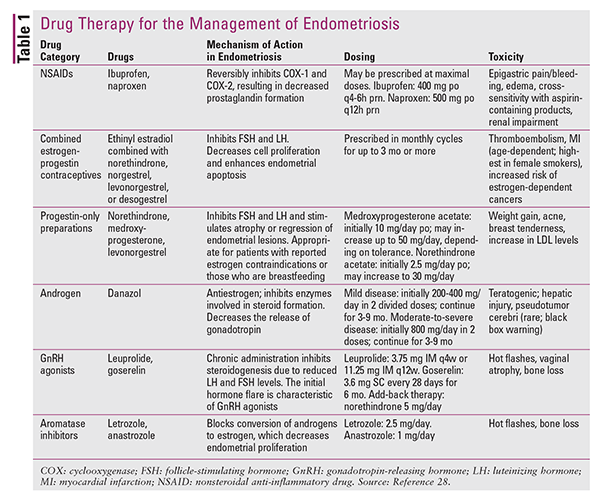
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): NSAIDs should be administered several days before the start of menstruation in order to block the endometriosis-associated prostaglandin formation that leads to pain and swelling.14 NSAIDs prevent prostaglandin E2 (PGE2) production through reversible blockade of COX-1 and COX-2. Nonprescription formulations (ibuprofen and naproxen) should be taken according to the manufacturer’s dosing instructions, and a prescription may be obtained from a physician if a higher dose is required. For the treatment of endometriosis, the highest NSAID dose tolerated by the patient should be administered. If the first NSAID is not effective after 4 to 6 weeks, another NSAID should be tried because of the variability in drug response among individuals. No NSAID has been observed to be superior to another.15 For the management of endometriosis, an NSAID may be combined with another pharmacologic agent, such as a hormonal preparation (discussed in upcoming sections).
Patients with a known aspirin allergy, bronchial asthma, and nasal polyps—often referred to as the aspirin triad—should avoid the use of NSAIDs, as cross-sensitivity reactions can occur.16 NSAIDs should be administered with plenty of water and on a full stomach. This is to protect the gastrointestinal tract from the loss of cytoprotective PGE2. Likewise, patients with a history of gastric irritation, ulceration, and bleeding should avoid COX-1 inhibitors. NSAIDs should not be administered on a chronic basis; renal injury can result from the loss of PGE2-mediated vasodilation in the afferent arteriole of the kidney, leading to a reduction in glomerular filtration rate through reduced blood flow. NSAIDs can also negate the effects of antihypertensives and the antiplatelet action of aspirin while increasing the toxicity of the mood stabilizer lithium.
Hormones: Combined estrogen-progestin contraceptives and progestin-only preparations reduce endometrial proliferation, blocking the inflammation and pain associated with endometriosis. The decreased ovulation afforded by these agents is protective against epithelial ovarian and endometrial cancers, which are associated with endometriosis.17 Gonadotropin-releasing hormone (GnRH) agonists, danazol, and aromatase inhibitors should also be considered.
Combined Estrogen-Progestin Contraceptives: These drugs, which are available in pill, transdermal patch, and vaginal ring formulations, are considered an appropriate treatment option for the pain of endometriosis until pregnancy is desired. Contraceptives reduce ovarian function by inhibiting the secretion of gonadotropins (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]). These drugs downregulate cell proliferation and enhance apoptosis in the eutopic endometrium.18 Serious adverse effects of estrogen-progestin combinations are lower than in previous years because the formulations contain a lower estrogen dose. However, the risks of thromboembolism, cerebrovascular disease, and coronary artery disease, which are attributed to the estrogen component of the formulation, cannot be eliminated. Smoking, especially in women older than 35 years, remains a contraindication to the use of combined estrogen-progestin contraceptives. It is also inadvisable for patients with a history of estrogen-dependent cancer to use these formulations. For the treatment of endometriosis, hormonal birth control should be administered continuously (skipping the placebo tablets) for 3 months or more in order to achieve less-frequent menstrual periods and consequently less pain.19
Progestin-Only Preparations: This therapy is appropriate for patients who do not respond to or have a contraindication to estrogen-progestin contraceptives. Progestins bind to nuclear receptors, leading to enhanced gene transcription and suppression of gonadotropin synthesis. In addition, progestins prevent the implantation and growth of regurgitated endometrial cells by inhibiting angiogenesis and matrix metalloproteinases.20 Norethindrone is started at 5 mg per day for 14 days and increased in increments of 2.5 mg per day to reach 15 mg per day, which is continued for 6 to 9 months or until the occurrence of breakthrough bleeding (when the uterine lining becomes thin). Medroxyprogesterone may be administered as an SC injection at a dosage of 150 mg every 3 months. In addition to breakthrough bleeding, progestin-only preparations can induce breast tenderness and acne. Weight gain is possible given the stimulation of insulin secretion and increased appetite.
Although limited results are available on the use of the levonorgestrel-releasing intrauterine system to treat endometriosis, this device has improved patient compliance over the once-daily oral progestin formulations because there is no repeated administration. Levonorgestrel enhances endometrial glandular atrophy and promotes endometrial apoptosis, resulting in a decrease in proliferation.21
Danazol: This agent is a synthetic androgen with minimal estrogen or progesterone potential. Danazol suppresses the activity of the ovary by inhibiting steroidogenic enzymes and preventing midcycle FSH and LH release. Because it can bind to glucocorticoid receptors, danazol also has been observed to modulate immunologic function.22 Danazol is administered orally in divided doses ranging from 400 mg to 800 mg daily for 6 to 9 months in mild cases; severe cases may require 800 mg per day in two divided doses. The increase in liver injury, masculinization, and risk of thromboembolism limits the use of danazol for endometriosis treatment. This drug is teratogenic and should be avoided in patients considering pregnancy. Abrupt discontinuation of danazol has been linked to idiopathic intracranial pressure (or pseudotumor cerebri), which may be due to a rebound phenomenon of cerebral vascular tone or prolonged effects of the drug’s metabolites.23
GnRH Agonists: These drugs bind to and downregulate pituitary gland receptors. The initial surge of LH and FSH may exacerbate endometriosis pain because of the ovarian stimulation. However, 2 weeks after therapy initiation, an estrogen-deficient state occurs; this is the mechanism of leuprolide and goserelin for endometriosis management. GnRH agonists also enhance apoptosis and decrease cellular proliferation in the endometrial cells.24 Some expected adverse effects are hot flashes, vaginal dryness, and atrophy. Since a hypoestrogenic state is induced, accelerated bone loss can occur in patients treated long-term with these agents. Therefore, an appropriate add-back regimen—the addition of a small amount of either estrogen and progesterone or progesterone alone—to lessen these toxicities should be considered. Leuprolide may be used in combination with norethindrone 3.75 mg per month for up to 6 months or norethindrone 11.25 mg every 3 months for up to two doses (total 6-month duration). Goserelin is administered at 3.6 mg every 28 days for 6 months.
Although further research is required, GnRH antagonists such as degarelix are being studied as possible management options for endometriosis. These drugs differ from GnRH agonists in that, instead of downregulation, there is a competitive blockade at the pituitary GnRH receptor. Gonadotropins are suppressed without the initial flare of estrogen.25
Aromatase Inhibitors: Aromatase activity is absent in the normal endometrium but is increased in patients with endometriosis.26 This enzyme is involved in the conversion of androstenedione to estrone and testosterone to estradiol. Increased estrogen production stimulates the synthesis of PGE2, leading to inflammation. Although considered an off-label use, aromatase inhibitors have been found beneficial for patients with postmenopausal endometriosis given the competitive blockade offered by letrozole and anastrozole. Long-term treatment with aromatase inhibitors has been associated with bone loss, so vitamin D or bisphosphonates are sometimes added to the regimen to prevent this deleterious effect. For the management of endometriosis, aromatase inhibitors are taken orally once daily and may be given concurrently with combined estrogen-progestin contraceptives, progestins, or GnRH agonists.
TABLE 1 summarizes the pharmacologic options recommended for endometriosis management.
Surgical Treatment: Surgical evaluation and treatment via laparoscopy is considered if drug therapy is unsuccessful, a pelvic mass is detected, or the patient is considering pregnancy. Conservative surgery is considered first-line treatment, as it is less invasive and preserves fertility because much of the uterus and ovary are preserved.27
Conclusion
Endometriosis is an estrogen-mediated growth of tissue outside the uterine lining that is characterized by recurring pain. The use of NSAIDs to manage inflammation and the administration of hormonal preparations to inhibit endometrial proliferation should be considered the main pharmacologic treatments for this condition. Pharmacists can play an important role in assessing the efficacy of these therapies, and they can evaluate endometriosis patients taking these medications for contraindications and adverse drug effects.
REFERENCES
1. Mettler L, Schmidt D, Maher P. The impact of endometriosis on the health of women 2016. Biomed Res Int. 2016;2016:1747280.
2. Slopien R, Meczekalski B. Aromatase inhibitors in the treatment of endometriosis. Prz Menopauzalny. 2016;15:43-47.
3. Culley L, Law C, Hudson N, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19:625-639.
4. Streuli I, Gaitzsch H, Wenger JM, Petignat P. Endometriosis after menopause: physiopathology and management of an uncommon condition. Climacteric. 2017;20:138-143.
5. Sampson JA. Heterotopic or misplaced endometrial tissue. Am Obstet Gynecol. 1925;10:649-664.
6. Augoulea A, Alexandrou A, Creatsa M, et al. Pathogenesis of endometriosis: the role of genetics, inflammation and oxidative stress. Arch Gynecol Obstet. 2012;286:99-103.
7. Sikora J, Mielczarek-Palacz A, Kondera-Anasz Z. Role of natural killer cell activity in the pathogenesis of endometriosis. Curr Med Chem. 2011;18:200-208.
8. Osuga Y, Koga K, Hirota Y, et al. Lymphocytes in endometriosis. Am J Reprod Immunol. 2011;65:1-10.
9. Christodoulakos G, Augoulea A, Lambrinoudaki I, et al. Pathogenesis of endometriosis: the role of defective ‘immunosurveillance’. Eur J Contracept Reprod Health Care. 2007;12:194-202.
10. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814-3826.
11. Borghese B, Zondervan KT, Abrao MS, et al. Recent insights on the genetics and epigenetics of endometriosis. Clin Genet. 2017;91:254-264.
12. King CM, Barbara C, Prentice A, et al. Models of endometriosis and their utility in studying progression to ovarian clear cell carcinoma. J Pathol. 2016;238:185-189.
13. Nisenblat V, Prentice L, Bossuyt PM, et al. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;(7):CD 012281.
14. Allen C, Hopewell S, Prentice A, Gregory D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
15. Brown J, Crawford TJ, Allen C, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2017;(1):CD004753.
16. Berkes EA. Anaphylactic and anaphylactoid reactions to aspirin and other NSAIDs. Clin Rev Allergy Immunol. 2003;24:137-148.
17. Vercellini P, Buggio L, Berlanda N, et al. Estrogen-progestins and progestins for the management of endometriosis. Fertil Steril. 2016;106:1552-1571.
18. Meresman GF, Augé L, Barañao RI, et al. Oral contraceptives suppress cell proliferation and enhance apoptosis of eutopic endometrial tissue from patients with endometriosis. Fertil Steril. 2002;77:1141-1147.
19. Davis L, Kennedy SS, Moore J, Prentice A. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(3):CD001019.
20. Vercellini P, Fedele L, Pietropaolo G, et al. Progestogens for endometriosis: forward to the past. Hum Reprod Update. 2003;9:387-396.
21. Viganò P, Somigliana E, Vercellini P. Levonorgestrel-releasing intrauterine system for the treatment of endometriosis: biological and clinical evidence. Womens Health (Lond). 2007;3:207-214.
22. Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;15;141:453-463.
23. Wu YS, Chen SD, Li TH, et al. Intracranial hypertension associated with danazol withdrawal: a case report. Acta Neurol Taiwan. 2007;16:173-176.
24. Meresman GF, Bilotas M, Buquet RA, et al. Gonadotropin-releasing hormone agonist induces apoptosis and reduces cell proliferation in eutopic endometrial cultures from women with endometriosis. Fertil Steril. 2003;80(suppl 2):702-707.
25. Küpker W, Felberbaum RE, Krapp M, et al. Use of GnRH antagonists in the treatment of endometriosis. Reprod Biomed Online. 2002;5:12-16.
26. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
27. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101:927-935.
28. Schenken RS. Endometriosis: treatment of pelvic pain. UpToDate. 2017.
To comment on this article, contact rdavidson@uspharmacist.com.





