US Pharm. 2015;40(12):34-38.
ABSTRACT: Motion sickness is a common malady in the general population; however, the exact mechanism through which it occurs is not completely known. Some people are more susceptible to motion sickness than others. Several effective pharmacologic treatments are available, including both prescription and OTC medications. Although there are several OTC options for motion sickness, it is important for pharmacists to consider individual patient characteristics before making a recommendation. All agents can cause unwanted side effects and have precautions for specific patient populations.
Motion sickness is a common occurrence for many people during travel. The brain normally receives signals from the inner ears, eyes, muscles, and joints. Motion sickness occurs when the motion perceived visually does not match that sensed by the brain.1 Motion sickness can manifest during air travel and in cars or trains, but it occurs most frequently during boat travel (seasickness). It can begin suddenly, with signs and symptoms including dizziness, nausea, and vomiting.2
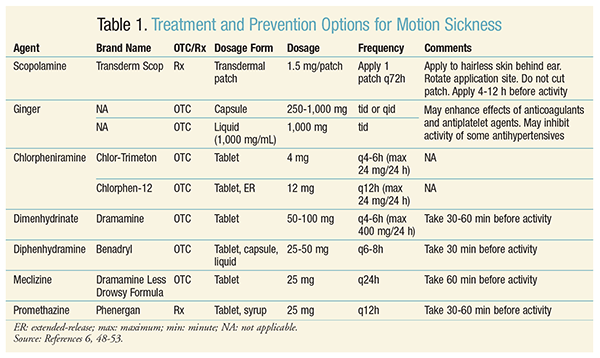
Susceptibility to motion sickness varies among individuals. Children aged 2 to 12 years are more susceptible to the condition than those aged <2 years. Women are more likely than men to experience motion sickness, especially during pregnancy or menstruation or in those taking hormone therapy. Patients with a history of migraines may be more likely to experience motion sickness. Additionally, motion sickness may be caused by certain medications, including but not limited to macrolides, metronidazole, morphine, digoxin, and some selective serotonin reuptake inhibitors.3,4 Numerous treatment and prevention options for motion sickness exist, as described in TABLE 1. Both pharmacologic and nonpharmacologic agents are available.
Scopolamine
Scopolamine is one of the most commonly prescribed medications for the treatment or prevention of motion sickness. This agent prevents motion sickness via its anticholinergic effects. Additionally, it decreases gastrointestinal secretions and motility and inhibits the secretion of saliva and sweat.5,6 Although scopolamine is available in several dosage forms, the most common form used for motion sickness is the transdermal patch.
Early studies were conducted in healthy volunteers who participated in the Spacelab missions in the 1970s and 1980s.7-9 Multiple studies were conducted to compare the scopolamine transdermal patch with placebo and antihistamines (dimenhydrinate, promethazine). In addition, various dosages of scopolamine were compared. In these trials, scopolamine was demonstrated to be superior to placebo for preventing motion sickness.
Comparisons of scopolamine and antihistamines have yielded varying results. Overall, the efficacy of scopolamine was found to be similar to that of dimenhydrinate and greater than that of promethazine.7-9 In sea studies including patients with and without a history of motion sickness, transdermal scopolamine was more efficacious than placebo for preventing motion sickness.10-13 Concerning the time of application, there was a greater benefit when the patch was applied 8 to 16 hours before motion exposure than when it was applied <4 hours beforehand.14,15
In a randomized, double-blind, placebo-controlled study, Pyykkö and colleagues evaluated the use of transdermal scopolamine and oral dimenhydrinate for treatment of motion sickness.16 Patients received one transdermal scopolamine patch, two transdermal scopolamine patches, oral dimenhydrinate 100 mg, or placebo. The scopolamine patches were applied 6 to 8 hours before motion exposure. Both scopolamine groups and the dimenhydrinate group experienced a greater reduction in nausea compared with the placebo group. Dimenhydrinate was more effective than the single scopolamine patch for reducing nausea. Adverse effects (AEs) in all groups were minimal, with gait disturbances occurring occasionally after application of two scopolamine patches.16
Price and colleagues compared transdermal scopolamine and dimenhydrinate for prevention of seasickness.14 Patients were given a transdermal scopolamine patch, oral dimenhydrinate, or placebo. The transdermal scopolamine patch was applied 4 to 16 hours before patients sailed on a ship for 7 to 8 hours; dimenhydrinate and placebo were administered 1.5 hours before motion exposure and 2.5 hours after motion began. Scopolamine and dimenhydrinate provided greater protection than placebo, but a direct comparison of scopolamine and dimenhydrinate was not completed. AEs associated with treatment included dry mouth, drowsiness, and blurred vision, and only dry mouth occurred more frequently with scopolamine than with dimenhydrinate.14
Dahl and colleagues conducted a study comparing scopolamine patches to meclizine 25 mg and placebo for prevention of motion sickness.9 Scopolamine patches were applied 12 hours before motion exposure, and oral tablets were taken 2 hours before exposure. Scopolamine subjects experienced less nausea compared with subjects given meclizine or placebo.
Finally, a Cochrane review of 14 randomized, controlled trials involving 1,025 patients evaluated the use of scopolamine for sea- or laboratory-induced motion sickness.17 Many comparators, including placebo, were assessed. Scopolamine provided a greater reduction in nausea compared with placebo; however, there was no difference in the occurrence of vomiting. In comparisons of scopolamine with antihistamines, two randomized, controlled trials determined scopolamine to be superior to meclizine and one randomized, controlled trial found scopolamine to be equivalent to dimenhydrinate.
Antihistamines
Antihistamines are commonly used to treat or prevent motion sickness. Since most of the agents in this class are OTC, they are easily accessible to the general public. Several antihistamines can be used to treat or prevent motion sickness. Specifically, antihistamines with central cholinergic blocking properties have proven efficacy in treating or preventing motion sickness.18 Thus, the anti–motion sickness effects of antihistamines are due not to the blockade of histamine1 receptors, but rather to their effects as central-acting anticholinergics.2,19 First-generation antihistamines are both peripheral and central-acting.20 Consequently, the antihistamines used to treat motion sickness are generally first-generation agents.
The most widely used medications in this class include diphenhydramine, dimenhydrinate (two of the most-studied motion-sickness drugs available in the U.S.), chlorpheniramine, meclizine, and promethazine.2,18,19 Second-generation antihistamines, such as cetirizine and fexofenadine, have been considered, but have not proven efficacious. This is likely because their central-acting properties are insufficient.21
In an evaluation of 16 anti–motion sickness drugs, Wood and Graybiel found that dimenhydrinate 50 mg was more effective than meclizine 50 mg.22 At low doses, chlorpheniramine has proven efficacy in preventing motion sickness, but its use is limited because its strong central effects result in excessive drowsiness.19 Meclizine, the longest-acting agent in this class, is widely used owing to its accessibility and marketing, although it has lower efficacy compared with other antihistamines and scopolamine, as described above in the study by Dahl and colleagues.9,18,19 Promethazine, which has the strongest antihistaminic and anticholinergic properties, is the most effective antihistamine in the class. Compared with scopolamine, promethazine is only slightly less effective in preventing motion sickness.23
Ginger Root
Although ginger is most commonly used as a culinary spice, it is believed to have many medicinal properties, including the treatment of motion sickness. Animal studies have concluded that ginger may enhance gastrointestinal transport and provide antiemetic effects.24 Ginger is also thought to affect gastric motility, antral contractions, and corpus motor response.25-29
A case report describes a reduction in disequilibrium and nausea with ginger root.30 Additionally, Grøntved and colleagues conducted a comparison of ginger root and placebo for prevention of seasickness.31 The population included naval cadets who were not accustomed to sailing on the high seas. Participants were given ginger 1 g or placebo. Ginger root resulted in reduced vomiting and cold sweats compared with placebo. Trends toward reductions in nausea and vertigo were seen, but statistical significance was not reached.31
Lien and colleagues compared two doses of ginger and placebo for the prevention of nausea associated with motion sickness in 13 patients with a history of motion sickness.32 Patients received ginger 1 g or 2 g or placebo before undergoing induced motion. Patients given ginger had less nausea, a prolonged time to nausea onset, and a shorter recovery time.
Ginger may cause diarrhea, gastric reflux, flatulence, and mouth irritation.24,33 Owing to ginger’s anticholinergic properties, caution should be exercised in elderly patients, patients with renal or hepatic impairment, and patients with pyloric, urinary/bladder, or intestinal obstruction.24,33 Ginger can result in drug interactions. It may enhance the effects of anticoagulants and antiplatelet agents, which can potentially result in toxicity, mainly bleeding. It may also inhibit the activity of some antihypertensive agents.34-37
Other Agents
Other pharmacologic agents have been investigated for the treatment of motion sickness, including sympathomimetics (e.g., d-amphetamine and ephedrine), other antiemetics, benzodiazepines, anticonvulsants, and tricyclic antidepressants.4,18,19,21 The mechanism by which sympathomimetics like amphetamine prevent motion sickness is unclear.19 Initially, it was thought that these agents increased the amount of norepinephrine in the central nervous system (CNS), which in turn countered the increased acetylcholine activity responsible for motion sickness.38 This notion was called into question after Takeda and colleagues proved that these medications do not increase CNS catecholamines.39 The latest theory targets the increase in dopamine.40
When used alone or in combination with scopolamine or promethazine, d-amphetamine is a viable prophylactic option.38 Owing to its stimulant properties, d-amphetamine also helps reduce the sedating effects of antihistamines or scopolamine.18 Ephedrine can be substituted for d-amphetamine as a noncontrolled option, but has shown less effective.41
The 5-HT3 (serotonin) blocker ondansetron has been studied for treatment and prevention of motion sickness based on its antiemetic effects in patients receiving chemotherapy. Two randomized, controlled trials involving patients with sea- or laboratory-induced motion sickness found no difference between ondansetron and placebo, however.42,43 Levine and colleagues have suggested that ondansetron may be ineffective because of its site of action, as it works at the vagal afferent receptor or the chemoreceptor trigger zone.44
A small number of studies have investigated the use of phenytoin for preventing motion sickness. Although the evidence appears promising, more studies must be performed before phenytoin can be determined a viable option.45,46 Lastly, the tricyclic antidepressant doxepin, which has strong antihistaminic, adrenergic, and anticholinergic effects, has been used to treat motion sickness. In a comparison of doxepin with a combination of scopolamine and amphetamine, Kohl and Lewis found doxepin alone to be as effective as the combination for preventing motion sickness.40
Conclusion
The treatment options discussed in this article have been studied to various degrees, with varying results. Several trials support the view that scopolamine is the most effective treatment option. While other options may be less effective, they are easily accessible and have proven helpful. Furthermore, it is important to keep individual patient characteristics in mind when a treatment option is being selected.
All treatment options have common, unwanted side effects that occur to various degrees. Because most of these medications work through anticholinergic effects, the most common AEs are drowsiness, dry mouth, dizziness, and blurred vision.18,19,47 In particular, scopolamine can also cause drowsiness, blurred vision, dilated pupils, euphoria, amnesia, and fatigue.5,6 The most common AE with the transdermal route is dry mouth. Although motion sickness occurs to varying degrees in different people and its etiology is not precisely known, several effective pharmacologic treatment options are available. Nonpharmacologic agents are appealing, but evidence supporting their use is inconsistent.
REFERENCES
1. MedlinePlus. Motion sickness. www.nlm.nih.gov/medlineplus/motionsickness.html. Accessed August 2, 2015.
2. Shupak A, Gordon CR. Motion sickness: advances in pathogenesis, prediction, prevention, and treatment. Aviat Space Environ Med. 2006;77:1213-1223.
3. CDC. Motion sickness. wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/motion-sickness. Accessed August 2, 2015.
4. Murdin L, Golding J, Bronstein A. Managing motion sickness. BMJ. 2011;343:d7430.
5. Scopolamine Injection (scopolamine) product information. Schaumburg, IL: APP Pharmaceuticals, LLC; April 2008.
6. Transderm Scop (scopolamine) product information. Princeton, NJ: Sandoz, Inc; April 2013.
7. McCauley ME, Royal JW, Shaw JE, Schmitt LG. Effect of transdermally administered scopolamine in preventing motion sickness. Aviat Space Environ Med. 1979;50:1108-1111.
8. Graybiel A. Prevention and treatment of space sickness in shuttle-orbiter missions. Aviat Space Environ Med. 1979;50:171-176.
9. Dahl E, Offer-Ohlsen D, Lillevold PE, Sandvik L. Transdermal scopolamine, oral meclizine, and placebo in motion sickness. Clin Pharmacol Ther. 1984;36:116-120.
10. Parrott AC, Jones R. Effects of transdermal scopolamine upon psychological test performance at sea. Eur J Clin Pharmacol. 1985;28:419-423.
11. van Marion WF, Bongaerts MC, Christiaanse JC, et al. Influence of transdermal scopolamine on motion sickness during 7 days’ exposure to heavy seas. Clin Pharmacol Ther. 1985;38:301-305.
12. Attias J, Gordon C, Ribak J, et al. Efficacy of transdermal scopolamine against seasickness: a 3-day study at sea. Aviat Space Environ Med. 1987;58:60-62.
13. How J, Lee PS, Seet LC, Tan PK. The Republic of Singapore Navy’s Scopoderm TTS study: results after 2,200 man-days at sea. Aviat Space Environ Med. 1988;59:646-650.
14. Price NM, Schmitt LG, McGuire J, et al. Transdermal scopolamine in the prevention of motion sickness at sea. Clin Pharmacol Ther. 1981;29:414-419.
15. Levy GD, Rapaport MH. Transderm scopolamine efficacy related to time of application prior to the onset of motion. Aviat Space Environ Med. 1985;56:591-593.
16. Pyykkö I, Schalén L, Jäntti V. Transdermally administered scopolamine vs. dimenhydrinate. I. Effect on nausea and vertigo in experimentally induced motion sickness. Acta Otolaryngol. 1985;99:588-596.
17. Spinks A, Wasiak J. Scopolamine (hyoscine) for preventing and treating motion sickness. Cochrane Database Syst Rev. 2011;(6):CD002851.
18. Sherman CR. Motion sickness: review of causes and preventive strategies. J Travel Med. 2002;9:251-256.
19. Zajonc TP, Roland PS. Vertigo and motion sickness. Part I: vestibular anatomy and physiology. Ear Nose Throat J. 2005;84:581-584.
20. Cheung B, Hofer K. Lack of gender difference in motion sickness induced by vestibular Coriolis cross-coupling. J Vestib Res. 2003;12:191-200.
21. Cheung BS, Heskin R, Hofer KD. Failure of cetirizine and fexofenadine to prevent motion sickness. Ann Pharmacother. 2003;37:173-177.
22. Wood CD, Graybiel A. Evaluation of sixteen anti-motion sickness drugs under controlled laboratory conditions. Aerosp Med. 1968;39:1341-1344.
23. Wood CD, Stewart JJ, Wood MJ, et al. Therapeutic effects of antimotion sickness medications on the secondary symptoms of motion sickness. Aviat Space Environ Med. 1990;61:157-161.
24. White B. Ginger: an overview. Am Fam Physician. 2007;75:1689-1691.
25. Micklefield GH, Redeker Y, Meister V, et al. Effects of ginger on gastroduodenal motility. Int J Clin Pharmacol Ther. 1999;37:341-346.
26. Phillips S, Hutchinson S, Ruggier R. Zingiber officinale does not affect gastric emptying rate. A randomised, placebo-controlled, crossover trial. Anaesthesia. 1993;48:393-395.
27. Mowrey DB, Clayson DE. Motion sickness, ginger, and psychophysics. Lancet. 1982;1:655-657.
28. Stewart JJ, Wood MJ, Wood CD, Mims ME. Effects of ginger on motion sickness susceptibility and gastric function. Pharmacology. 1991;42:111-120.
29. Shariatpanahi ZV, Taleban FA, Mokhtari M, Shahbazi S. Ginger extract reduces delayed gastric emptying and nosocomial pneumonia in adult respiratory distress syndrome patients hospitalized in an intensive care unit. J Crit Care. 2010;25:647-650.
30. Schechter JO. Treatment of disequilibrium and nausea in the SRI discontinuation syndrome. J Clin Psychiatry. 1998;59:431-432.
31. Grøntved A, Brask T, Kambskard J, Hentzer E. Ginger root against seasickness. A controlled trial on the open sea. Acta Otolaryngol. 1988;105:45-49.
32. Lien H-C, Sun WM, Chen Y-H, et al. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am J Physiol Gastrointest Liver Physiol. 2003;284:G481-G489.
33. Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12:684-701.
34. Mousa SA. Antithrombotic effects of naturally derived products on coagulation and platelet function. Methods Mol Biol. 2010;663:229-240.
35. Stanger MJ, Thompson LA, Young AJ, Lieberman HR. Anticoagulant activity of select dietary supplements. Nutr Rev. 2012;70:107-117.
36. Spolarich AE, Andrews L. An examination of the bleeding complications associated with herbal supplements, antiplatelet and anticoagulant medications. J Dent Hyg. 2007;81:67.
37. Ulbricht C, Chao W, Costa D, et al. Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr Drug Metab. 2008;9:1063-1120.
38. Wood CD, Manno JE, Manno BR, et al. The effect of antimotion sickness drugs on habituation to motion. Aviat Space Environ Med. 1986;57:539-542.
39. Takeda N, Morita M, Yamatodani A, et al. Catecholaminergic responses to rotational stress in rat brain stem: implications for amphetamine therapy of motion sickness. Aviat Space Environ Med. 1990;61:1018-1021.
40. Kohl RL, Lewis MR. Mechanisms underlying the antimotion sickness effects of psychostimulants. Aviat Space Environ Med. 1987;58:1215-1218.
41. Tokola O, Laitinen LA, Aho J, et al. Drug treatment of motion sickness: scopolamine alone and combined with ephedrine in real and simulated situations. Aviat Space Environ Med. 1984;55:636-641.
42. Muth ER, Elkins AN. High dose ondansetron for reducing motion sickness in highly susceptible subjects. Aviat Space Environ Med. 2007;78:686-692.
43. Hershkovitz D, Asna N, Shupak A, et al. Ondansetron for the prevention of seasickness in susceptible sailors: an evaluation at sea. Aviat Space Environ Med. 2009;80:643-646.
44. Levine ME, Chillas JC, Stern RM, Knox GW. The effects of serotonin (5-HT3) receptor antagonists on gastric tachyarrhythmia and the symptoms of motion sickness. Aviat Space Environ Med. 2000;71:1111-1114.
45. Chelen W, Kabrisky M, Hatsell C, et al. Use of phenytoin in the prevention of motion sickness. Aviat Space Environ Med. 1990;61:1022-1025.
46. Stern RM, Uijtdehaage SH, Muth ER, Koch KL. Effects of phenytoin on vection-induced motion sickness and gastric myoelectric activity. Aviat Space Environ Med. 1994;65:518-521.
47. Golding JF. Motion sickness susceptibility. Auton Neurosci. 2006;129:67-76.
48. Ginger. In: Natural Product Database [by subscription]. Hudson, OH: Wolters Kluwer; 2015.
49. Chlor-Trimeton Allergy 4 Hour (chlorpheniramine maleate) product information. Memphis, TN: MSD Consumer Care, Inc; July 2013.
50. Dramamine (dimenhydrinate) product information. Irvington, NY: Medtech Products, Inc; April 2012.
51. Benadryl (diphenhydramine) product information. Fort Washington, PA: McNeil Consumer Healthcare; May 2015.
52. Dramamine Less Drowsy Formula (meclizine) product information. Irvington, NY: Medtech Products Inc; August 2011.
53. Promethazine product information. Glasgow, KY: Amneal Pharmaceuticals; February 2012.
To comment on this article, contact rdavidson@uspharmacist.com.





