US Pharm. 2011;36(7):HS3-HS8.
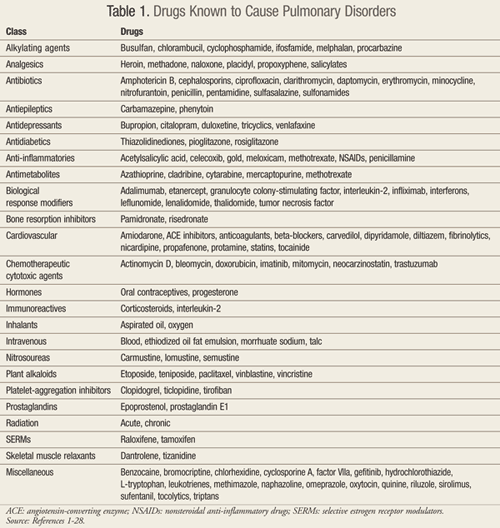
Many drugs, including herbal products, have been implicated as the cause of pulmonary adverse events.1 However, the definitive diagnosis of drug-induced pulmonary disorder (DIPD) remains challenging as the clinical presentation is nonspecific and there are no confirmatory diagnostic tests. As such, DIPD is typically a diagnosis of exclusion.1,2 This review will provide an update regarding medications and their potential for pulmonary side effects. A summary of drugs known to cause pulmonary disorders, although not all inclusive, is provided in TABLE 1.1-28
In 1880, Osler first described heroin-induced pulmonary edema during an autopsy.3,4 In the early 1900s, drugs were increasingly identified as causing pulmonary syndromes. Between 1920 and 1930, it was first suspected that aspirin could induce severe asthma attacks and death. In the 1940s, antibiotics and gold were identified as causes of pulmonary interstitial disease.5 In the 1960s, medication-induced pulmonary hypertension was first identified as a consequence of the appetite-suppressant aminorex.6 History repeated itself when two anorectic agents, dexfenfluramine and fenfluramine (Fen-Phen), were withdrawn from the U.S. market in 1997 due to multiple reports of drug-induced pulmonary hypertension.1
It is apparent that new drugs are more likely to produce adverse pulmonary effects that are similar to those previously reported with agents from the same pharmacologic category. Examples include the angiotensin-converting enzyme (ACE) inhibitors and cough, beta-blockers and bronchospasm, or the nonsteroidal anti-inflammatory drugs (NSAIDs) and bronchospasm or eosinophilic pneumonia.7
Foral et al published an extensive review of DIPD that cited over 70 agents.1 Since then, the list of products implicated in DIPD has grown. One useful reference is www.pneumotox.com; this free, regularly updated resource systematically grades the evidence that a given drug is responsible for a specific lung disease.8
Existing studies attempting to quantify the frequency of drug-induced adverse pulmonary reactions are limited by inconsistencies in the patient populations studied, lack of a standardized definition, method(s) of detection, and self-reporting bias. As such, it is difficult to draw convincing conclusions on the epidemiology of this disorder.1,29-31 However, it is reported that approximately 0.5% to 1.2% of patients experiencing an adverse drug reaction manifest respiratory symptoms.2 While relatively uncommon, pulmonary adverse reactions are of marked clinical importance as they often lead to significant physiologic impairment and/or necessitate intervention.2
Pathogenesis
Little information is available regarding the precise mechanism(s) of DIPD. However, such reactions can affect not only the pulmonary parenchyma but also the pleura, airways, pulmonary vasculature, and/or respiratory muscles.1 Theoretic mechanisms include: 1) cytotoxic effects on alveolar capillary endothelial cells; 2) direct oxidative injury; 3) amphophilic medications causing deposition of phospholipid within the cells—particularly the alveolar macrophage; and 4) immune-mediated lung injury, either through drug-induced systemic lupus erythematosus (SLE) or via hypersensitivity reactions.1
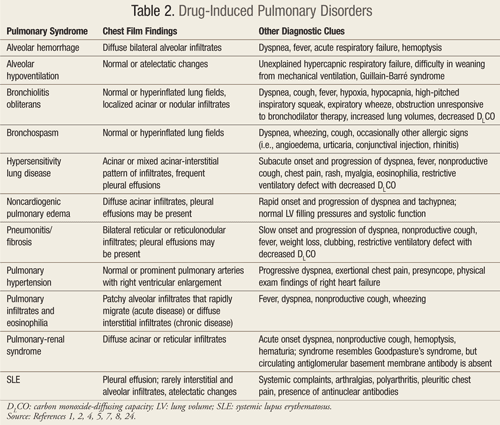
These mechanisms of injury result in a variety of clinical and histologic patterns associated with drug-induced pulmonary syndromes, including pneumonitis, fibrosis, hypersensitivity reaction, noncardiogenic pulmonary edema, bronchospasm, and pulmonary hypertension. Each of these classic syndromes can be caused by more than one medication, and many drugs can cause more than one syndrome.1 TABLE 2 highlights the clinical features of these common drug-induced pulmonary syndromes.1,2,4,5,7,8,24
Typical Drug-Induced Pulmonary Syndromes
Pneumonitis and Fibrosis: Symptoms of pneumonitis and fibrosis include dyspnea and minimally productive cough that slowly progress over weeks to months.1 Agents implicated in this syndrome are extensive and include adalimumab, amiodarone, beta-blockers, bleomycin, busulfan, carmustine, ciprofloxacin, dantrolene, etanercept, hydralazine, lomustine, methotrexate, nitrofurantoin, oxygen, radiation, statins, sulfasalazine, tamoxifen, and thalidomide.1,2,4,7,8,12,16,
Long-term use of nitrofurantoin to treat urinary tract infections is a classic cause of pulmonary fibrosis.25 The prescribing information for nitrofurantoin capsules warns of acute, subacute, and chronic pulmonary reactions; diffuse interstitial pneumonitis; and/or pulmonary fibrosis.26 Acute interstitial pneumonitis has recently been reported as a side effect of thalidomide when used in combination with topotecan for the treatment of ovarian cancer.27 When thalidomide was discontinued in these patients, symptoms immediately resolved.
Hypersensitivity Lung Disease: Hypersensitivity lung disease differs from pneumonitis/fibrosis in that the onset is quicker, usually occurring over days to weeks.1 Symptoms include fever, rash, and myalgias, which almost always respond promptly to discontinuation of the offending agent with or without concurrent corticosteroid therapy.1
Statin-related hypersensitivity pneumonitis (particularly with pravastatin, lovastatin, and simvastatin) has increasingly been reported in the literature. This remains a clinical diagnosis as histology on lung biopsy is not uniformly confirmatory.9,23 Ticlopidine has also recently been associated with hypersensitivity pneumonitis: symptoms resolved with discontinuation of the drug and prednisone therapy.11 Another case report implicated ciprofloxacin as the cause of interstitial pneumonitis leading to acute respiratory failure.12 Discontinuation of the drug and treatment with oral corticosteroids led to rapid improvement. Sirolimus-induced granulomatous hypersensitivity reaction with interstitial pneumonitis and organizing pneumonia is another newly reported association. Drug withdrawal was followed by rapid clinical and radiologic improvement.10 There are many drugs that can cause hypersensitivity lung disorders besides the above mentioned. These include ampicillin, bupropion, carbamazepine, cephalosporins, cytarabine, erythromycin, interferon alfa, methotrexate, nitrofurantoin, NSAIDs, penicillin, phenytoin, sulfonamides, and trimethoprim-sulfamethoxazole (TMP-SMX).1,2,4,7-12,23,24
Noncardiogenic Pulmonary Edema: This condition typically develops within hours after exposure and resolves quickly after discontinuation of the drug involved. Common causes include sympathomimetics, narcotics, and salicylates.1 Thiazolidinedione-induced congestive heart failure has also been observed in two case reports, one involving pioglitazone and the other rosiglitazone.20 Additional drugs that can cause noncardiogenic pulmonary edema include carbamazepine, cytarabine, erythromycin, hydrochlorothiazide, IV radiographic contrast agents, methotrexate, protamine, tamoxifen, and tumor necrosis factor.1-4,7,8,15,20,24
Bronchospasm: Classes of drugs known to cause bronchospasm include NSAIDs, aspirin, and beta-blockers. Patients with asthma and chronic obstructive pulmonary disease (COPD) are particularly prone to this phenomenon; it has been reported that up to 20% of asthmatic patients cannot tolerate aspirin and other NSAIDs.28 Drug delivery by any route (oral, IV, inhaled, topical) can lead to this potentially life-threatening event. Undiagnosed reactive airway disease may be unmasked by exposure to these agents.1 Bronchospasm can also be caused by ACE inhibitors, cephalosporins, contrast material, hydrocortisone, nitrofurantoin, penicillin, propafenone, and tamoxifen, to name a few.1,2,4,7,8,24,25,28
Atypical Drug-Induced Pulmonary Syndromes
In addition to the classic pulmonary syndromes described above, several atypical drug-induced pulmonary syndromes have been described. These include SLE, bronchiolitis obliterans, alveolar hemorrhage, eosinophilic reactions, hypoventilation, and pulmonary-renal syndrome.1
Systemic Lupus Erythematosus: Although pulmonary manifestations of drug-induced SLE are diverse, pleuritis presenting with pleural effusion and pleuritic chest pain is reported by 50% to 80% of patients. A positive antinuclear antibody (ANA) titer is common and confirms the diagnosis. Hydralazine, isoniazid, phenytoin, procainamide, and sulfonamides are the most commonly cited causes (TABLE 3).1,2,4,7,8,13,14,17-19,24 Symptoms rapidly resolve with discontinuation of the offending agent, and the length of time to recovery is shortened by corticosteroid administration.1

Bronchiolitis Obliterans: This condition is characterized by small airway inflammation, granulation tissue formation, and varying degrees of secondary airways obstruction. This rare syndrome is associated with a limited number of drugs (sulfasalazine and penicillamine) and a handful of clinical conditions (severe viral respiratory infections, autologous bone marrow transplants, severe inhalation injuries, and rheumatoid arthritis).1 The apparent interaction between penicillamine and rheumatoid arthritis resulting in bronchiolitis obliterans merits further study with other drugs and disease states (TABLE 3).1,2,4,7,8,13,14,17-19,24
Alveolar Hemorrhage: Gefitinib is a chemotherapeutic agent with antitumor activity against a number of human cancer cells expressing epidermal growth factor receptor (EGFR; e.g., non–small cell lung, ovarian, breast, and colon cancers). Although it is well tolerated, it is also associated with multiple adverse drug reactions. A recent case report implicated gefitinib as the cause of alveolar hemorrhage when this agent was used to treat lung cancer.14 Discontinuation of the drug, empiric high-dose methylprednisolone, and supportive care led to gradual improvement in the patient’s respiratory status (TABLE 3).1,2,4,7,8,13,14,17-19,24
Eosinophilic Reactions: Several drugs are reported causes of eosinophilic pleural effusion, and diltiazem has recently been added to this list (TABLE 3).1,2,4,7,8,13,14,17-19,24 In one case report, symptoms started soon after initiation of diltiazem and subsided quickly after its discontinuation.19
Eosinophilic pneumonia can be induced by several drugs, particularly venlafaxine. This entity has a slow progression; pulmonary infiltrates and eosinophilia typically progress over weeks and resolve upon withdrawal of the offending agent.13 Duloxetine has also been reported to cause eosinophilic pneumonia.17 Hayes et al recently reported a case of drug-induced eosinophilic pneumonia caused by daptomycin.18
Hypoventilation: Drug-induced alveolar hypoventilation can manifest via multiple avenues, including depression of the respiratory drive, peripheral motor neuropathies, a Guillain-Barré–like syndrome, myopathy, or potentiation of neuromuscular blockade.1,2 Common offending drugs include alcohol, sedatives, narcotics, and hypnotics. Glucocorticoids, calcium channel blockers, select antibiotics (especially aminoglycosides), and muscle relaxants are reported to induce and/or prolong neuromuscular blockade.1 Caution should be used when administering any of these agents to patients with myasthenia gravis due to an increased risk of respiratory depression.1 TABLE 4 lists some of the agents that can result in hypoventilation.1,2,4,7,8,24

Pulmonary–Renal Syndrome: This syndrome is a rare complication of penicillamine therapy that presents with acute onset of rapidly progressive dyspnea, hematuria, hemoptysis, and pleuritic chest pain. Onset of pulmonary manifestations may be temporally removed from initiation of drug therapy; case reports describe initial symptoms after more than 2 years of penicillamine therapy. Note that although pulmonary–renal syndrome clinically resembles Goodpasture’s syndrome, the two can be distinguished through measurement of the circulating antiglomerular basement membrane antibody, which is not present in drug-induced pulmonary–renal syndrome.1
Alternative Medicine Products
Pharmacists and other health professionals should be mindful of the potential dangers of nutrition supplements and alternative medicines. Because many of these products have not been studied in large populations, there is little if any published information available on the potential toxicity of these agents. Reported adverse reactions involving herbal products most frequently include herbal–drug interactions. There have also been many public warnings about adulterated herbal products. Accordingly, the Dietary Supplement Health and Education Act established new policies for the regulation of herbal products within the FDA.32 This regulation differs from that used for food and drugs in that there is no requirement for premarketing safety testing. As a result, the burden of identifying dangerous products falls on the FDA, not the manufacturer. These changes are welcome as multiple-ingredient products containing agents that are concentrated many times over the natural concentrations are increasingly common.33 To date, the only existing documented pulmonary toxicity from herbal therapy involves 10 cases of interstitial pneumonia reportedly caused by the Chinese medicine Sho-saiko-to.1
Illicit Drug Use
The lungs can also be damaged by injection or inhalation of illicit drugs. Vascular congestion and pulmonary edema are the most common findings in users who died within 3 hours of overdose. In contrast, lobar pneumonia was the predominant finding in those who died more than 12 hours after the overdose. Pulmonary edema and hypoxic respiratory failure are the classic complications of heroin overdose.15 Talc or hydrous magnesium silicate used to cut drugs for illicit injectable drug use lead to foreign-body pulmonary embolism, pulmonary granulomatosis, progressive massive fibrosis, and pulmonary hypertension. Frequent symptoms include cough, dyspnea, and hemoptysis.4 Crack cocaine lung disease or “crack lung” is a distinct clinical syndrome. Pleurisy, cough, wheezing, dyspnea, hemoptysis, and upper airway thermal burns are some of the symptoms. Other findings include increased levels of ferritin and iron, which may contribute to chronic lung disease. Cocaine-induced pulmonary edema, alveolar hemorrhage, and acute hypersensitivity reactions may also occur.4
Role of the Pharmacist
Diagnosing and treating DIPDs continues to present obstacles for clinicians. These conditions are commonly overlooked, as they closely mimic other pulmonary conditions. Pharmacists can play a significant role in completing a detailed medication history (prescription drugs, OTCs, herbal products, and illicit drug use) in such situations where a patient presents with a potential drug reaction. The safety profile of a medication may change over time, and all health professionals should regularly monitor each drug their patients are taking. Pharmacists can take an active role in identifying those patients who have underlying risk factors (e.g., pulmonary disease, heart failure, advanced age) that may increase their risk for DIPD.
Reporting of suspected adverse drug reactions has made a difference as the FDA relies on health professionals for spontaneous reporting. The FDA’s MedWatch program encourages and facilitates clinicians in reporting suspected adverse drug reactions. MedWatch offers a one-page reporting form (FDA Form 3500) that health professionals may utilize when reporting adverse events with medications, dietary supplements, or problems with devices or products regulated by the FDA. MedWatch can be contacted by phone (800-FDA-1088), fax (800-FDA-0178), or via the Internet (
www.fda.gov/safety/medwatch/
REFERENCES
1. Foral PA, Malesker MA, Dewan NA, et al. Drug-induced pulmonary disorders. US Pharm. 1999;24(7):HS3-HS19.
2. Raissy HH, Harkins M, Marshik PL. Drug-induced pulmonary disease. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York: McGraw-Hill; 2008:521-534.
3. Sternbach G. William Osler: narcotic-induced pulmonary edema. J Emerg Med. 1983;1:165-167.
4. Bhadra K, Suratt B. Drug-induced lung disease: a state-of-the-art review. J Resp Dis. 2009;30:1-17.
5. Camus PH. Respiratory disease induced by drugs. Eur Respir J. 1997;10:260-264.
6. Fishman AP. Aminorex to Fen/Phen: an epidemic foretold. Circulation. 1999;99:156-161.
7. Foucher P, Biour M, Blayac P, et al. Drugs that may injure the respiratory system. Eur Respir J. 1997;10:265-279.
8. Foucher P, Camus PH, Groupe d’Etudes de la Pathologie Pulmonaire Iatrogène (GEPPI). The drug-induced lung diseases. Pneumotox Online. June 17, 2010.
www.pneumotox.com. Accessed August 4, 2010.
9. Lantuejoul S, Brambilla E, Brambilla C, et al. Statin-induced fibrotic nonspecific interstitial pneumonia. Eur Respir J. 2002;19:577-580.
10. Howard L, Gopalan D, Griffiths M, et al. Sirolimus-induced pulmonary hypersensitivity associated with CD4 T-Cell infiltrate. Chest. 2006;129:1718-1721.
11. Persoz C, Cornella F, Kaeser P, et al. Ticlopidine-induced interstitial pulmonary disease: a case report. Chest. 2001;119:1963-1965.
12. Steiger D, Bubendorf L, Oberholzer M, et al. Ciprofloxacin-induced acute interstitial pneumonitis. Eur Respir J. 2004;23:172-174.
13. Fleisch M, Blauer F, Gubler J, et al. Eosinophilic pneumonia and respiratory failure associated with venlafaxine treatment. Eur Respir J. 2000;15:205-208.
14. Leki R, Saitoh E, Shibuya M. Acute lung injury as a possible adverse drug reaction related to gefitinib. Eur Respir J. 2003;22:179-181.
15. Wolff AJ, O’Donnell AE. Pulmonary effects of illicit drug use. Clin Chest Med. 2004;25:203-216.
16. Camus P, Martin II W, Rosenow III E. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25:65-75.
17. Espeleta V, Moore W, Kane P, et al. Eosinophilic pneumonia due to duloxetine. Chest. 2007;
131:901-903.
18. Hayes D Jr, Anstead M, Kuhn R. Eosinophilic pneumonia induced by daptomycin. J Infect. 2007;54:e211-e213.
19. Raptis L, Pappas G, Katsanou A, et al. Diltiazem-induced eosinophilic pleural effusion. Pharmacotherapy. 2007;27:600-602.
20. Cheng A, Fantus I. Thiazolidinedione-induced congestive heart failure. Ann Pharmacother. 2004;
38:817-820.
21. Yonemaru M, Mizuguchi Y, Kasuga I, et al. Hilar and mediastinal lymphadenopathy with hypersensitivity pneumonitis induced by penicillin. Chest. 1992;102:1907-1909.
22. Thornburg A, Abonour R, Smith P, et al. Hypersensitivity pneumonitis-like syndrome associated with the use of lenalidomide. Chest. 2007;131:1572-1574.
23. Liebhaber M, Wright R, Gelberg H, et al. Polymyalgia, hypersensitivity pneumonitis and other reactions in patients receiving HMG-COA reductase inhibitors: a report of ten cases. Chest. 1999;115:886-889.
24. Camus P, Foucher P, Bonniaud P, et al. Drug-induced infiltrative lung disease: Eur Respir J. 2001;18(suppl 32):93s-100s.
25. Goemaere N, Grijim K, van Hal P, et al. Nitrofurantoin-induced pulmonary fibrosis: a case report. J Med Case Rep. 2008;2:169.
26. Nitrofurantoin package insert. Sellersville, PA: Teva Pharmaceuticals USA; March 2009.
27. Buttin B, Moore M. Thalidomide-induced reversible interstitial pneumonitis in a patient with recurrent ovarian cancer. Gynecol Oncol. 2008;111:546-584.
28. Babu K, Salvi S. Aspirin and asthma. Chest. 2000;118:1470-1476.
29. American Society of Health-System Pharmacists. ASHP guidelines on adverse drug reaction monitoring and reporting. Am J Health Syst Pharm. 1995;52:417-419.
30. Bond C, Raehl C. Adverse drug reactions in United States hospitals. Pharmacotherapy. 2006;26:601-608.
31. Kongkaew C, Noyce P, Ashcroft D. Hospital admissions associated with adverse reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42:1017-1025.
32. Dietary supplements. FDA.
www.fda.gov/food/
33. Smolinski SG. Herbal product contamination and toxicity. J Pharm Pract. 2005;18:188-208.
34. MedWatch: the FDA Safety and Adverse Event Reporting Program.
www.fda.gov/safety/medwatch/
To comment on this article, contact rdavidson@uspharmacist.com.






