US Pharm. 2007;32(9):HS-12-HS-15.
Pregnancy
has been identified as a condition prone to thrombosis and embolism, although
there is limited published information regarding treatment options for
thrombosis in pregnant women.1 Pregnancy could be considered as the
initiation of a hypercoagulable state that lasts for a period of 11 months--10
months of pregnancy and one month postpartum.2 Due to the
physiologic changes that occur in pregnancy, the coagulation cascade's
response is altered in order to minimize blood loss during gestation and
delivery. While hemorrhage and embolism are common causes of death in
pregnancy, venous thromboembolism is a leading cause of maternal deaths.
3,4 Compared to nonpregnant women, the risk of venous thromboembolism
during pregnancy and after delivery is increased about fivefold.5
Thromboembolic complications in the United States occur in about 1 in 1,000
pregnancies and in approximately 1 in 5,000 deliveries.4-6
Thrombolytic agents available
in the U.S. are alteplase (tissue plasminogen activator [t-PA], recombinant
t-PA [rt-PA]), reteplase, streptokinase, urokinase, and tenecteplase.
Currently, clinical guidelines regarding the use of thrombolytic agents in
pregnant women who present with an active clot are nonexistent. Yet, numerous
case reports have been published regarding the use of these agents in pregnant
women. This article provides a brief overview of the use of thrombolytic
agents in pregnant women. Indirect (e.g., low-molecular-weight heparin and
heparin) and direct (e.g., argatroban, bivalirudin, and lepirudin) thrombin
inhibitors will not be discussed.
Etiology of Thrombosis in
Pregnancy
Increased levels of
estrogen and certain coagulation factors are key components in the development
of a hypercoaguable state during pregnancy.4 These elements can
lead to venous distension, potentially resulting in venous stasis, and
increased levels of coagulation factors with a subsequent increased rate of
activity.7 These mechanisms are thought to be the cause of the
higher rates of deep vein thrombosis (DVT), pulmonary embolism (PE), stroke,
and thrombosis of cardiac valvular prosthesis in pregnancy.8-10
Untreated DVT may result in PE in up to 24% of pregnant patients, with an
associated mortality rate of approximately 15%.4 In addition,
pregnancy has been shown to cause a significant decrease in the production of
natural anticoagulant protein S and an increase in the development of acquired
activated protein C resistance.7,11
There are three components,
known as Virchow's triad (VT), that can lead to the formation of
a thrombus: blood vessels, circulating elements in the blood, and the speed of
blood flow.12 An irregularity of any component of the VT may cause
thrombosis and embolism, which can reduce blood flow to critical organs.1
Mechanisms and Adverse
Effects of Thrombolytic Therapy
Contraindications
to the use of thrombolytic agents in pregnancy is relative to the patient, and
consideration of the benefits versus the risks of treatment is an important
step prior to administration. All thrombolytic agents work by activating
plasminogen, which ultimately results in the degradation of fibrin clots. The
human fibrinolytic system stimulates the release of tissue plasminogen
activator from endothelial cells in response to various signals or injuries.
Tissue plasminogen activator binds to plasminogen within the clot and converts
to plasmin. The enzyme plasmin (a nonspecific protease) digests fibrin
(protein that forms a "mesh") clots and several coagulation factors. The human
fibrinolytic system is regulated in such a way that unwanted fibrin clots are
removed from circulation while simultaneously leaving desired fibrin in wounds
intact to maintain hemostasis.1,12,13 Therefore, clot dissolution
is known as a product of fibrin degradation. Plasminogen and plasmin bind to
fibrin at binding sites located near the amino termini that are located in the
lysine residues. The lysine residues are also a site for alpha-2-antiplasmin,
which covalently binds to fibrin, thereby protecting it from premature lysis.
When plasminogen activators are administered for thrombolytic therapy, massive
fibrinolysis is initiated and the inhibitory controls are overwhelmed.13
Exogenously administered thrombolytic agents not only dissolve the
pathological thrombi but also dissolve the surrounding fibrin deposits at the
site of injury.
Thus, the major side effect of
thrombolytic administration is hemorrhage.12 The use of
thrombolytics may cause several complications. Major adverse events include
life-threatening hemorrhage (i.e., uterine and postpartum), intracranial
hemorrhage, and incomplete clot dissolution that may lead to embolization.
12,13
The ideal thrombolytic agent
would induce local pathological clot dissolution without producing systemic
fibrinolysis or disrupting physiologic thrombi necessary for normal hemostatic
balance.14 Since there is no ideal thrombolytic agent, a variety of
adjunctive therapies (e.g., aspirin, clopidogrel, and heparin) are required to
achieve all of the hemostatic goals.14 Because aspirin and
clopidogrel are not indicated for use in pregnant patients, heparin or
low-molecular-weight heparin are the only options for thrombus prophylaxis.
It is difficult to choose a
thrombolytic therapy for a pregnant patient when the package insert indicates
a pregnancy category C and the major adverse event is hemorrhage. There is
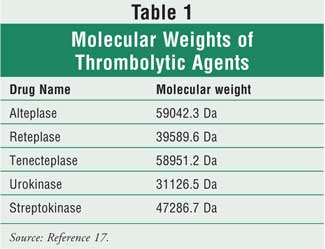
always the concern of entry of the drug into the placenta. However, several
factors influence the transfer of drugs to the placenta, such as molecular
weight, lipid solubility, drug pH, and binding to plasma proteins. Molecular
weight is the most important factor to consider when addressing the use of
thrombolytic agents in pregnancy. Drugs with molecular weights greater than
1,000 Da often poorly cross the placenta.15 Thus, thrombolytic
agents are unlikely to cross the placenta due to their large molecular weight
16 (see Table 1).
Diagnosis of Clotting
Disorders During Pregnancy
Early diagnosis of
clotting disorders is important to prevent complications. Most diagnostic
procedures have excluded pregnant women; therefore, it is difficult to
formulate an evidence-based recommendation.
DVT begins in the calf or in
the ileofemoral segment, with a tendency to occur in the left leg due to
compression of the iliac vein by the gravid uterus (uterus containing a
developing fetus). Noninvasive studies, such as impedance plethysmography (the
measurement of blood volume changes) and ultrasonography, are recommended as
initial diagnostic tools for clots in pregnancy. However, if isolated iliac
vein thrombosis is suspected and if the veins cannot be identified by
ultrasonography or venography, a more complex and invasive procedure could be
considered.18
PE can be difficult to
diagnose, especially in pregnant women. Symptoms of PE should be interpreted
with caution during pregnancy because dyspnea, tachypnea, and chest discomfort
are already common. Electrocardiogram, chest radiographs, and arterial blood
gas may support a PE diagnosis. In addition, a lung scan, also known as a
ventilation perfusion scan, is the primary screening tool for the
diagnosis in pregnant patients but exposes the fetus to radiation (although
the amount has not been associated with significant risk of fetal injury).
18
Stroke, the loss of brain
function due to interruption of blood supply, is a risk factor in pregnant
patients. A pregnant woman experiences natural changes from an elevation in
blood pressure and stress on the heart, which increases the risk of stroke.
Common clinical presentations of stroke symptoms include headache, focal
neurologic deficit, seizures, and visual changes. Clinicians should perform a
neurologic examination, a funduscopic examination to assess intracranial
pressure, as well as cardiovascular and skin examinations. In addition, tests
such as CT and MRI may be performed.10 However, the use of MRI
during the first trimester should be avoided.18
Risk Factors for Venous
Thromboembolism in Pregnant Patients
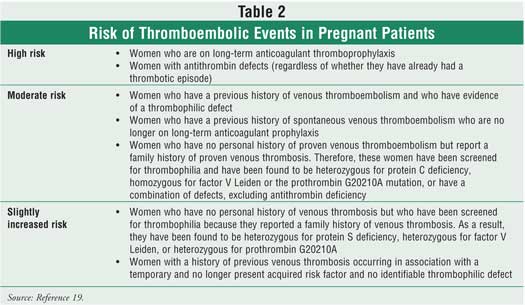
The management of
pregnant women with known thrombophilic defects and no prior history of
thromboembolism remain controversial. Information is limited regarding the
natural history of the various thrombophilia, and there is a lack of
appropriate trials for prophylaxis. Pregnant women with previous thrombosis
events may be at risk of a recurrent event. Women with a history of venous
thromboembolism and inherited thrombophilia (i.e., protein C and S deficiency,
factor V Leiden, prothrombin G20210A, and antithrombin) may be classified as
being at a high, moderate, or slightly increased risk of thromboembolic events
during pregnancy (Table 2).19
Case Reports
Since there are no
existing randomized, controlled trials to evaluate the use of thrombolytics
agents during pregnancy, cases reported in the literature will be reviewed.
In a case report by Yap et
al., a 28-year-old woman in week 30 of her pregnancy who presented with PE
received rt-PA 10 mg IV bolus, followed by 90 mg over two hours. The patient's
hemodynamic status improved. A healthy baby boy was born by vaginal delivery
at 36 weeks' gestation.20
In a report by Patel et al., a
36-year-old woman presented at 20 weeks' gestation with a five-day history of
worsening breathlessness, chest pain, and palpitations. The diagnosis of
massive PE was made, and rt-PA was administered at 10 mg bolus, followed by a
90 mg infusion over two hours. There was an improvement of blood pressure from
67/40 to 120/80 mmHg. After discontinuing thrombolytic therapy, she received
unfractionated heparin for 48 hours, followed by low-molecular-weight heparin.
The fetal outcome was not reported.21
In a case reported by Flobdorf
et al., a 27-year-old pregnant woman at 31 weeks' gestation was admitted to
the hospital with respiratory distress and tachypnea. She was diagnosed with
massive PE. Initial treatment was urokinase, but this medication was switched
to rt-PA 10 mg over four hours, followed by 2 mg over 1.5 hours. Forty-eight
hours after thrombolysis, the patient delivered a healthy infant. Maternal
outcomes were reported as stable.22
In another report by Ahearn et
al., a 12-week pregnant, 36-year-old African-American woman presented with
acute dyspnea and substernal chest pain in the emergency department. The
patient was lethargic with a blood pressure of 82/50 mmHg and a pulse of 135
beats per minute. She was diagnosed with massive PE. The patient received 100
mg of rt-PA over two hours. Maternal outcomes were reported to be stable, and
the patient delivered a full-term infant without complications.23
A case reported by Elford et
al. describes a 28-year-old, newly gravid woman with a seven-year history of
primary infertility and four unsuccessful cycles of ovulation induction. The
patient presented with dysarthria, left facial paralysis, and drowsiness; she
was diagnosed with a stroke that was confirmed by CT. The patient was
administered 15.5 mg of intra-arterial rt-PA. Over a three-week period, the
patient regained hematologic and neurologic stability. She delivered a healthy
male infant at term by vaginal delivery.24
Murugappan et al. reported on
eight pregnant women diagnosed with acute ischemic stroke who were treated
with either rt-PA or urokinase. The average maternal age was 32 years and the
mean gestational age was 11 weeks (range, 4-37 weeks). Etiologies of stroke
were varied (e.g., discontinuation of anticoagulants in patients with
mechanical heart valves, hypercoaguable states). Four women were treated with
rt-PA, and the other four were treated with urokinase. One of the mothers died
secondary to complications of angioplasty. This death was determined by the
authors to not be caused solely by thrombolytic therapy. Of the mothers who
survived, three elected to have therapeutic abortions, two miscarried within
two to three days of urokinase administration, and two mothers delivered at
term without any complications (one received rt-PA and the other received
urokinase).25
A 27-year-old pregnant woman
in her first trimester was hospitalized due to pregnancy complications for
three weeks. Her clinical condition deteriorated and she developed shock,
followed by cardiac arrest. The diagnosis was consistent with acute PE, and
the patient received streptokinase. This treatment was complicated by massive
bleeding due to the rupture of the uterus. She underwent hysterectomy and
recovered thereafter. No further information was provided on the patient's
condition.26
Role of the Pharmacist
As the
pathophysiology of thrombus formation becomes increasingly understood, new
therapeutic options have emerged and the role of existing pharmacotherapeutic
agents has expanded. Each thrombolytic agent has its own specific dosing
regimen, typically based on the clotting disorder being treated. Pharmacists
can have an integral role in drug selection based on patient-specific
parameters. In addition, therapeutic monitoring is essential for adequate and
sustained patient care in order to avoid adverse effects. This includes
monitoring for any sign of bleeding, allergic reactions, and in some
instances, improvement for clinical outcomes. Through close patient
interaction and attention to specific symptoms, pharmacists can help patients
avoid potential complications and achieve appropriate goals.
Conclusion
The majority of
cases presented in this article resulted in encouraging outcomes. In one case,
the use of hormones for fertility was thought to be the cause of thrombus
formation. This substantiates the documented risk of hormone use in the
development of thromboembolic events and illustrates the need for pharmacists
to be aware of the increased risk of thrombosis in women who use fertility
agents.7,13 In terms of maternal mortality, all cases presented
positive outcomes. Regarding fetal outcomes, one case did not report the
outcome, while most of the remaining cases reported positive fetal outcomes.
It is interesting to note that rt-PA was the drug of choice to treat a
pregnant patient in the cases reported. The reason for this decision could be
that rt-PA has the least potential to cause antigenicity and has the largest
molecular weight in comparison to all the other thrombolytic agents.14
Generally, the use of
thrombolytic therapy during pregnancy cannot be recommended until further
safety and efficacy data are available. However, based on the cases assessed
in this article, it appears that the use of a thrombolytic agent, particularly
rt-PA, may be an effective therapeutic option for pregnant women who present
with life-threatening blood clots.
References
1. Handin R. Hematologic alterations. In: Harrison's Principles of Internal Medicine. [book online]. The McGraw-Hill. 2005. Available at: www.accessmedicine.com.novacat.nova.edu. Accessed May 3, 2007.
2. Toglia MR, Weg JG. Venous thromboembolism during pregnancy. N Engl J Med. 1996;335:108-114.
3. MacKay AP, Berg CJ, Duran C, et al. An assessment of pregnancy-related mortality in the United States. Paediatr Perinat Epidemiol.2005;19:206-214.
4. Doyle N, Monga M. Thromboembolic disease in pregnancy. Obstet Gynecol Clin North Am. 2004;31:319-344.
5. Berg CJ, Atrash HK, Koonin LM, et al. Pregnancy related mortality in the United States, 1987-1990. Obstet Gynecol. 1996;88:161-167.
6. Lestsky W, Swiet M. Maternal hemostasos: coagulation problems of pregnancy. In: Loscalzo J, Schafer A, editors. Thrombosis and Hemorrhage. Boston, MA: Blackwell Scientific, 1994:965-998.
7. Bendell J, Benz EJ. Hematologic changes in pregnancy. In: Hoffman R, Benz Jr, Shanttil SJ, et al., editors. Hematology: Basic Principal and Practice. 4th ed.. Philadelphia, PA: Churchill Levingstone; 2005.
8. Leonhardt G, Gasul C, Nietsch HH, et al. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis. 2006;21:271-276.
9. Chan WS, SDC, Ginsberg JS. Antithrombotic therapy during pregnancy. Semin Perinatol. 2001;25:165-169.
10. Turan TN, Stern B. Stroke in pregnancy. Neurol Clin. 2004;22:821-840.
11. Gordon MC. Maternal physiology in pregnancy. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 4th ed. Philadelphia, PA: Churchill Levingstone; 2002:63-91.
12. Majerus PW, Broze Jr GJ, Miletich JP, Tollefsen DM. Anticoagulant, thrombolytic, and antiplatelet drugs. In: Dipiro J, Talbert RL, Yee JC, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 3rd ed. Stamford, CT: McGraw-Hill; 1997.
13. Haines ST, Racine E, Zeolla M. Venous thromboembolism. In: Dipiro J, Talbert RL, Yee JC, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 5th ed. Stamford, CT: McGraw-Hill, 2002.
14. Baker W. Thrombolytic therapy Clinical applications. Hematol Oncol Clin N Am. 2003;17:283-311.
15. Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487-514.
16. Briggs GG, Freeman RK., Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. [electronic resource]. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
17. Wishart DS, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;1:34.
18. Toglia MR, Weg JG. Venous thromboembolism during pregnancy. N Engl J Med. 1996;335:108-114.
19. Walker ID, Greaves M, Preston FE. Investigation and management of heritable thrombophilia. Br J Haematol . 2001;114:512-528.
20. Yap LB, Alp NG, Forfar JC. Thrombolysis for acute massive pulmonary embolism during pregnancy. Int J Cardiol. 2002;82:193-194. Letter.
21. Patel RK, Fasan O, Arya R. Thrombylsis in pregnancy. Thromb Haemost. 2003;90:1216-1217. Letter.
22. Flobdorf TH, Breulmann M, Hopf H. Successful treatment of massive pulmonary embolism with recombinant tissue plasminogen activator (rt-PA) in a pregnant woman with intact gravity and preterm labour. Intensive Care Med. 1990;16:454-456.
23. Ahearn GS, Hadjiliadis D, Govert JA , et al. Massive pulmonary embolism during pregnancy successfully treated with recombinant tissues plasminogen activator. Arch Intern Med. 2002;162:1221-1227.
24. Elford K, Leader A, Wee R, et al. Stroke in ovarian hyperstimulation syndrome in early pregnancy treated with intra-arterial rt-PA. Neurology. 2002;59:1270-1272.
25. Murugappan A, Coplin WM, Al-Sadat AN, et al. Thrombolytic therapy for acute ischemic stroke during pregnancy. Neurology. 2006;66:678-770.
26. Kukla P, Borowicz J, Szczuka K,
et al. Massive pulmonary embolism during pregnancy treated with streptokinase
and complicated by massive hemorrhage -a case report. Kardiol Pol.
2004;60:505-509. [Abstract]
To comment on this article, contact
uspharmacist.com.





