US Pharm. 2016;41(7)(Specialty&Oncology suppl):3-7.
ABSTRACT: Treatments for certain types of cancer have advanced significantly in the last few years, particularly those for non–small-cell lung cancer, multiple myeloma, and melanoma. New oral chemotherapy options are now available for treatment of these cancers. These agents offer patients multiple advantages, including increased convenience, improved quality of life, and a reduction in the use of healthcare resources. Pharmacists should understand the key benefits and safety concerns of these agents in order to help patients and their healthcare providers optimize therapy with these novel agents.
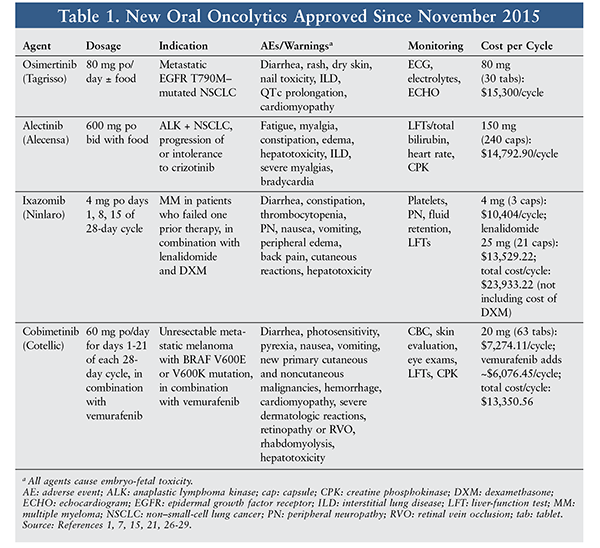
Several oral chemotherapy agents have been recently approved by the FDA for patients with certain types of cancer. Oral oncolytics offer several advantages over traditional parenteral chemotherapy, including added convenience in treating an already-complex disease state, which can improve quality of life and compliance. Four new oral chemotherapy drugs will be discussed, with a focus on non–small-cell lung cancer (NSCLC), multiple myeloma (MM), and melanoma. See TABLE 1.
NSCLC
Osimertinib: Osimertinib (Tagrisso) (TABLE 1) was recently approved for epidermal growth factor receptor (EGFR) T790M mutation–positive (+) NSCLC patients who have progressed on or after tyrosine kinase inhibitor (TKI) therapy.1 Osimertinib gained accelerated approval from the FDA based on data from a study designed to evaluate safety and efficacy.2 In a cohort study of patients with locally advanced or metastatic NSCLC with a known EGFR TKI-sensitizing mutation or prior clinical benefit, osimertinib dosages of 20 mg to 240 mg per day were tested. Dose-expansion cohorts were opened if the dosage had an acceptable toxicity profile, and patients in the dose-expansion cohorts were required to undergo EGFR mutation testing. Of the 253 patients who received at least one dose of the study drug, 62% were T790M+ and 80% had had prior chemotherapy. In patients with confirmed EGFR T790M disease, 61% had an objective response, and 95% had disease control. Of those with a confirmed response, 85% to 88% had a duration of response of ≥6 months. Median progression-free survival (PFS) was 9.6 months in T790M+ responders versus 2.8 months in T790M-negative patients with only 30% maturity.2
The most common adverse events (AEs) with osimertinib were diarrhea, rash, nausea, and diminished appetite.1,2 Diarrhea and rash incidence increased in a dose-dependent fashion. AEs of grade 3 or higher occurred in 32% of patients; dose reductions were made in 7% of patients, and therapy was discontinued in 6%. Serious AEs included pneumonitis, hyperglycemia, and QTc prolongation. Of the fatal AEs that occurred in seven patients, one case involved pneumonia attributable to the drug. AEs are graded based on severity (grades 1-5) in accordance with the Common Terminology Criteria for Adverse Events developed by the National Cancer Institute. Grade 1 AEs are the mildest and grades 4 and 5 are the most severe, with grade 5 signifying AE-related death.
Osimertinib received accelerated approval based on these data; however, several questions remain. NSCLC metastasizes to the brain, and given the immature data, the possibility of central nervous system (CNS) penetration and efficacy remains unknown. Long-term safety and efficacy studies have yet to be completed, but some are currently enrolling participants. The AURA2 trial is assessing the safety and efficacy of osimertinib in patients with metastatic NSCLC with the T790M mutation following failure of an EGFR TKI.3 Several other studies will address the appropriate sequence of TKI therapy. AURA3 will investigate treatment following failure of an EGFR TKI compared with a platinum-based doublet.4 The FLAURA study is currently comparing osimertinib to gefitinib or erlotinib in treatment-naïve patients with advanced NSCLC with an EGFR-sensitizing mutation.5,6 These studies will provide further evidence regarding osimertinib’s place in therapy.
Alectinib: Alectinib (Alecensa) (TABLE 1) was approved in late 2015 for use in metastatic, anaplastic lymphoma kinase+ (ALK+) NSCLC in patients who have progressed on or are intolerant to crizotinib.7 Accelerated approval was based on safety and efficacy studies in locally advanced or metastatic ALK+ NSCLC.8,9 A global, single-arm, phase II study of the safety and efficacy of alectinib in ALK+ lung cancer enrolled 138 patients, 61% of whom had CNS metastases; 75% of these had prior brain radiation.9 All patients were treated with alectinib 600 mg orally twice daily. The primary endpoint was objective response rate (ORR), and secondary endpoints included PFS, overall survival, and CNS efficacy.9 The ORR was 50%, with a median duration of response (DOR) of 11.2 months. Of evaluable patients, 79% had received prior chemotherapy and had an ORR of 45%. The median PFS was 8.9 months overall. CNS efficacy was demonstrated with an ORR of 57%, a CNS control rate of 83% with a complete CNS response in 27%, and a median DOR of 10.3 months.9
A second single-arm, multicenter, open-label, phase II study evaluated alectinib in 87 patients with locally advanced or metastatic ALK+ NSCLC.8 More than one-half of patients had baseline CNS metastases and three-quarters had a history of prior chemotherapy. The primary endpoint was ORR; secondary endpoints were objective response in the CNS, disease control in the CNS, CNS progression, DOR, and PFS. A partial response was confirmed in 48% of patients with measurable disease. Objective response, as determined by the independent review committee, was achieved by 48% of patients. The median PFS was estimated at 8.1 months. The median duration of CNS response was 11.1 months, and 89% achieved disease control. Of the 52 patients with CNS disease, 18 patients had no prior history of brain radiation; of these, 12 (67%) had an objective response, demonstrating activity in patients with CNS metastases without prior radiation.
Overall, alectinib was well tolerated; the most common AEs were constipation, fatigue, peripheral edema, and myalgia.7-9 Most AEs were grade 1 or 2, and the incidence of grade 3 and 4 AEs was low. Dose interruptions were required in up to one-third of patients, and one-fifth had dose reductions as a result of toxicity.8,9 Treatment interruptions and dose reductions most frequently were secondary to laboratory abnormalities of aspartate aminotransferase, alanine aminotransferase, and creatine phosphokinase (CPK). Warnings and precautions regarding the use of alectinib include hepatotoxicity, interstitial lung disease, bradycardia, severe myalgias, CPK elevations, and embryo-fetal toxicity.7
These two studies led to the accelerated approval of alectinib for ALK+ NSCLC following progression on or intolerance to crizotinib.8,9 Despite the evidence for CNS efficacy, several questions remain: 1) How should next-generation ALK inhibitors be chosen, and 2) should mutational status drive treatment decisions?10 Also, 3) in treatment-naïve ALK+ NSCLC, is there an advantage to treating with alectinib prior to crizotinib? The ALEX study is an active phase III clinical trial comparing crizotinib and alectinib in newly diagnosed ALK+ NSCLC in the first-line setting.11 Another trial is evaluating the combination of alectinib and bevacizumab in ALK+ NSCLC with untreated or asymptomatic brain metastases.12 Alectinib is also being compared with single-agent chemotherapy in a phase III clinical trial in ALK+ NSCLC patients with prior crizotinib and platinum-containing chemotherapy exposure.13 Another area being investigated is how the combination of ALK inhibitors and immunotherapy may affect outcomes of patients with ALK+ NSCLC.
MM
Several new chemotherapy agents have recently been approved for MM, including proteasome inhibitors and immunomodulatory drugs. Whereas previously MM was largely considered a fatal disease with an average survival of about 3 years, these newer agents show much promise for long-term control and extended survival.14
Ixazomib: Ixazomib (Ninlaro) (TABLE 1) was approved in 2015 for the treatment of MM in patients who received at least one previous therapy. Ixazomib is a reversible proteasome inhibitor that, when given in combination with lenalidomide and dexamethasone, has been proven safe and effective in patients in whom other therapies have failed.15 Studies are being conducted to evaluate this combination in patients with previously untreated MM or with limited prior treatment.16
The safety and efficacy of ixazomib given in combination with lenalidomide and dexamethasone was examined in a randomized, placebo-controlled trial of 722 patients. Ixazomib 4 mg or placebo was administered on days 1, 8, and 15 of a 28-day cycle, along with lenalidomide 25 mg on days 1 through 21 and dexamethasone 40 mg on days 1, 8, 15, and 22. The ixazomib group showed a statistically significant improvement in PFS (hazard ratio [HR] 0.74, 95% CI 0.59-0.94; P = .012), with a median duration of response of 20.6 months versus 14.7 months in the placebo group.17
Other Agents: Other available proteasome inhibitors include bortezomib (the prototype proteasome inhibitor) and carfilzomib. Bortezomib significantly improves survival, but it must be given SC and has major AEs, particularly neuropathy and diarrhea; carfilzomib must be given IV twice weekly. Therefore, ixazomib offers definite advantages as an oral once-weekly drug. Although the various proteasome inhibitors have a similar AE profile, fewer patients reported neuropathy in studies evaluating ixazomib’s safety.14 Other serious AEs include thrombocytopenia, gastrointestinal AEs, hepatotoxicity, rash, and peripheral edema. Platelet counts and hepatic enzymes should be monitored during treatment. Dose adjustment may be an appropriate management strategy for ixazomib-related AEs of lesser severity.15
The treatment of MM has advanced significantly since the 1960s, when the combination of melphalan plus prednisone was introduced and demonstrated the first improvements in median survival. The genomic complexity of MM means that multiple mechanisms of action must be utilized in the development of treatment regimens. In addition to further advances with proteasome inhibitors, other treatments—such as histone deacetylase inhibitors, immune therapies, monoclonal antibodies, and vaccines—are in development.18
Melanoma
Metastatic melanoma poses a significant treatment challenge, and the prognosis is often poor. Genomic evaluation determined that about 50% of melanomas have a BRAF V600 mutation, which led to the development of BRAF inhibitors such as vemurafenib. However, because of the paradoxical activation of the mitogen-activated protein kinase (MAPK) pathway in keratinocytes with BRAF inhibition, secondary squamous cell carcinomas and keratoacanthomas occur in approximately 20% of patients. Additionally, tumor resistance is likely to occur with monotherapy. The addition of an MEK (defined below) inhibitor, such as cobimetinib, can block MAPK activation, thereby preventing these secondary cutaneous cancers.19 Dual therapy is also more likely to delay melanoma treatment resistance and is therefore preferable in most cases.20
Cobimetinib: Cobimetinib (Cotellic; TABLE 1) was approved in 2015 for the treatment of unresectable or metastatic melanoma with a BRAF V600E or V600K mutation in patients also taking vemurafenib. Cobimetinib is a reversible inhibitor of MAPK/extracellular signal regulated kinase 1 (MEK1) and MEK2 that ultimately prevents cellular proliferation and tumor cell growth.21
The efficacy and safety of cobimetinib were investigated in a randomized, placebo-controlled trial of 495 patients with previously untreated BRAF V600 mutation+ unresectable or metastatic melanoma. All patients received vemurafenib and were randomized to cobimetinib 60 mg or placebo once daily on days 1 to 21 of every 28-day cycle. PFS was significantly improved in cobimetinib-plus-vemurafenib patients versus placebo-plus-vemurafenib patients (HR 0.51, 95% CI 0.39-0.68; P <.001). Median PFS was 9.9 months in the treatment group versus 6.2 months in the placebo group.19
The most common AEs associated with cobimetinib are nausea, vomiting, and diarrhea. Photosensitivity reactions and pyrexia are also prevalent, and patients should be counseled to avoid sun exposure. Liver enzymes and CPK should be evaluated during treatment and as indicated for suspicion of hepatotoxicity or rhabdomyolysis.21
Combination therapy with cobimetinib for metastatic melanoma offers significant advantages over single-agent therapy with vemurafenib. AEs with dual therapy, although common, are not significantly increased compared with AEs with vemurafenib monotherapy. Further evaluation of strategies to prevent tumor resistance and to optimize vemurafenib-cobimetinib therapy will strengthen the use of these agents as the standard of care for metastatic melanoma.20
Further Considerations
Given the significant increase in the number of oral oncolytics over the last several years, gaps in procedures for safe management and monitoring of oral chemotherapy regimens remain.22-24 The use of oral chemotherapy and its incorporation into treatment regimens is increasing, providing several advantages for patients.22,23 Oral agents offer patients more control, increased convenience, improved quality of life, lower travel costs, and a reduction in the use of healthcare resources.22 Although these advantages benefit both the patient and the healthcare system, many safety concerns exist regarding oral chemotherapy regimens administered at home. Oral chemotherapy has a narrow therapeutic index, increased AEs, a greater risk of errors, the potential for decreased adherence, and often a lack of coordinated care, which results in more AEs.22,23 The cost of oral chemotherapy, unintentional exposure to family, and disposal of oral chemotherapy are additional safety concerns that must be addressed with each patient. A 2007 survey showed variations in oral chemotherapy prescribing, coordination of care, and monitoring, as well as underutilization of pharmacy services.25 In 2009, the American Society of Clinical Oncology and the Oncology Nursing Society developed guidelines and standards for the use and monitoring of oral chemotherapy. These standards, which were revised in 2013, provide guidance for healthcare providers on staffing, chemotherapy planning, general chemotherapy practice standards, ordering/prescription standards, drug preparation, chemotherapy administration, consent and education, and monitoring and assessment.24 As the number of oral oncolytics increases in the coming years, it is important that the benefits and risks of using oral agents be understood by patients, families, and providers.
References
1. Tagrisso (osimertinib) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; November 2015.
2. Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689-1699.
3. ClinicalTrials.gov. Phase II AZD9291 open label study in NSCLC after previous EGFR TKI therapy in EGFR and T790M mutation positive tumours (AURA2). https://clinicaltrials.gov/ct2/show/NCT02094261. Accessed June 6, 2016.
4. ClinicalTrials.gov. AZD9291 versus platinum-based doublet-chemotherapy in locally advanced or metastatic non-small cell lung cancer (AURA3). https://clinicaltrials.gov/ct2/show/NCT02151981. Accessed June 6, 2016.
5. Gil-Bazo I, Rolfo C. AZD9291 in TKI EGFR resistance in non-small cell lung cancer and the new concept of phase I trials. Transl Lung Cancer Res. 2016;5:85-88.
6. ClinicalTrials.gov. AZD9291 versus gefitinib or erlotinib in patients with locally advanced or metastatic non-small cell lung cancer (FLAURA). https://clinicaltrials.gov/ct2/show/NCT02296125. Accessed June 6, 2016.
7. Alecensa (alectinib) product information. South San Francisco, CA: Genentech Inc; December 2015.
8. Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661-668.
9. Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234-242.
10. Sullivan I, Planchard D. ALK inhibitors in non-small cell lung cancer: the latest evidence and developments. Ther Adv Med Oncol. 2016;8:32-47.
11. ClinicalTrials.gov. ALEX study: a randomized, phase III study comparing alectinib with crizotinib in treatment-naive anaplastic lymphoma kinase-positive advanced non-small cell lung cancer participants. https://clinicaltrials.gov/ct2/show/NCT02075840. Accessed June 6, 2016.
12. ClinicalTrials.gov. Phase I/II trial of alectinib and bevacizumab in patients with advanced, anaplastic lymphoma kinase (alk)-positive, non-small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT02521051. Accessed June 6, 2016.
13. ClinicalTrials.gov. Alectinib versus pemetrexed or docetaxel in anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) participants previously treated with platinum-based chemotherapy and crizotinib. https://clinicaltrials.gov/ct2/show/NCT02604342. Accessed June 6, 2016.
14. Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503-1512.
15. Ninlaro (ixazomib) product information. Cambridge, MA: Millennium Pharmaceuticals, Inc; November 2015.
16. Kumar SK, LaPlant B, Roy V, et al. Phase 2 trial of ixazomib in patients with relapsed multiple myeloma not refractory to bortezomib. Blood Cancer J. 2015;5:e338.
17. ClinicalTrials.gov. A phase 3 study comparing oral ixazomib plus lenalidomide and dexamethasone versus placebo plus lenalidomide and dexamethasone in adult patients with relapsed and/or refractory multiple myeloma. http://clinicaltrials.gov/show/NCT01564537. Accessed June 6, 2016.
18. Bianchi G, Richardson PG, Anderson KC. Promising therapies in multiple myeloma. Blood. 2015;126:300-310.
19. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
20. Richman J, Martin-Liberal J, Diem S, Larkin S. BRAF and MEK inhibition for the treatment of advanced BRAF mutant melanoma. Expert Opin Pharmacother. 2015;16:1285-1297.
21. Cotellic (cobimetinib) product information. South San Francisco, CA: Genentech USA, Inc; May 2016.
22. Goodin S, Griffith N, Chen B, et al. Safe handling of oral chemotherapeutic agents in clinical practice: recommendations from an international pharmacy panel. J Oncol Pract. 2011;7:7-12.
23. Aisner J. Overview of the changing paradigm in cancer treatment: oral chemotherapy. Am J Health Syst Pharm. 2007;64(9 suppl 5):S4-S7.
24. Neuss MN, Polovich M, McNiff K, et al. 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. J Oncol Pract. 2013;9(suppl 2):S5-S13.
25. Weingart SN, Flug J, Brouillard D, et al. Oral chemotherapy safety practices at US cancer centres: questionnaire survey. BMJ. 2007;334:407.
26. Osimertinib. Lexicomp Online [online database]. Hudson, OH: Wolters Kluwer Health, Inc; 2015. http://online.lexi.com. Accessed March 15, 2016.
27. Alectinib. Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2016. http://online.lexi.com. Accessed March 15, 2016.
28. Ixazomib. Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2016. http://online.lexi.com. Accessed March 15, 2016.
29. Cobimetinib. Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2016. http://online.lexi.com. Accessed March 15, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.





