US
Pharm. 2006;31(4)(Oncology suppl):3-10.
Myelodysplastic syndrome
(MDS) is a collection of disorders that is difficult to manage clinically, as
the advanced age of patients at diagnosis renders the administration of
therapy challenging. Appropriate treatment options for MDS range from
supportive care through blood transfusions or colony-stimulating factors to
intensive therapy with chemotherapy or allogeneic stem cell transplantation
(alloSCT). Currently, therapies in clinical studies, including lenalidomide,
azacitidine, and decitabine, appear promising for the treatment of this
disease.
MDS is a heterogeneous group
of clonal hematologic disorders characterized clinically and morphologically
by ineffective hematopoiesis. This process can lead to varying degrees and
combinations of anemia, neutropenia, and thrombocytopenia, which may place
patients with the disease at risk for infection, bleeding, and dependence on
red blood cell transfusions.1 In addition, MDS can progress to
acute myeloid leukemia (AML) in approximately one third of patients.2
It is estimated that 15,000 to 20,000 new cases of MDS are diagnosed annually
in the United States.3 The median age at diagnosis of the disease
is 60 to 75 years. Due to increases in average life expectancy and growing
awareness of MDS, incidence of the disease is expected to rise in the next
decade.3,4
ETIOLOGY AND RISK FACTORS
MDS is the most
common hematologic disease among the elderly and occurs in a greater
proportion of men than women.1 Exposure to certain chemicals has
been associated with MDS; agents such as benzene have a clear association with
MDS, while smoking tobacco is weakly associated with development of the
disease.5 Exposure to antineoplastic alkylating agents and ionizing
radiation has been shown to have a clear association with MDS, which usually
develops four to seven years after initial exposure.5 The majority
of cases of MDS (80% to 90%) are idiopathic (de novo). MDS arising from
chemotherapy or ionizing radiation is referred to as secondary, or
therapy-induced MDS.2,5 This distinction is important since
secondary MDS carries a poorer prognosis compared to de novo MDS.6
A small percentage of patients may have genetic factors leading to MDS, also
known as familial MDS.5
SIGNS AND SYMPTOMS
Patients with MDS
may present with signs and symptoms of hematopoietic failure, such as
infection, bleeding, bruising, petechiae, pallor, progressive fatigue, or
dyspnea on exertion.1 Lymphadenopathy and hepatosplenomegaly are
infrequent.7 Many patients present without any symptoms, but rather
with incidental findings of anemia, thrombocytopenia, leuko penia, or a
combination of these on routine laboratory evaluations.1
Initial diagnosis of MDS is
made by determining the peripheral blood counts and performing a careful
microscopic analysis of the peripheral blood cells. Other crucial diagnostic
tests include cytochemistry of bone marrow cells, immuno phenotyping,
cytogenetics, in vitro characteristics of bone marrow, and molecular genetics.
5 Additional causes of abnormal hematopoiesis, such as vitamin B12
or folate deficiency, aplastic anemia, or human immunodeficiency virus
infection, should also be ruled out.
CLASSIFICATION
Different systems
have been used to classify MDS. Classification based on the
French-American-British (FAB) system consists of five subgroups of MDS: (1)
refractory anemia (RA); (2) refractory anemia with ringed sideroblasts (RARS);
(3) refractory anemia with excess blasts (RAEB); (4) refractory anemia with
excess blasts in transformation (RAEB-t); and (5) chronic myelomonocytic
leukemia (CMML). Each subgroup is differentiated by the number of ringed
sideroblasts (erythroblasts containing cytoplasmic iron granules arranged in a
ring around the nucleus), degree of monocytosis, and percentage of myeloblasts
(blasts) in the bone marrow and peripheral blood (table 1).8 An
increased number of blasts, which are immature blood cells, may be indicative
of leukemia.1 According to this classification system, patients
were diagnosed with MDS when the blast percentage was less than 30% and with
AML when the blast percentage was greater than 30%.8 As MDS was
increasingly recognized as a hematologic disorder, it became apparent that not
all patients could be easily classified into one of the FAB subgroups.
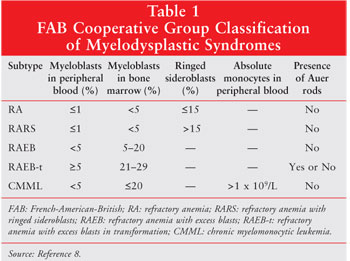
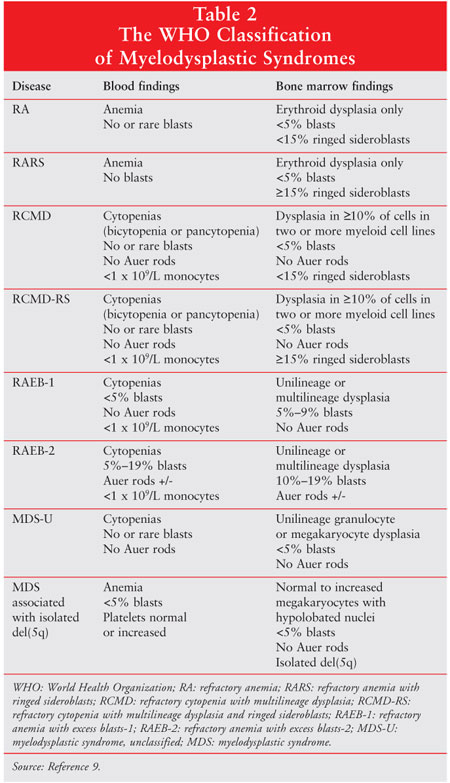
Using the FAB system as a framework, the World Health Organization (WHO) developed a revised classification system for MDS (table 2).9 Undoubtedly, the most important change in this new system was the lowering of the blast count required for MDS diagnosis from less than 30% to less than 20%. Although the WHO criteria for staging may provide more prognostic information as compared to the FAB system, even the most recent clinical studies continue to incorporate the FAB classification criteria.
STAGING AND PROGNOSIS
The International
Prognostic Scoring System (IPSS) is the most widely used grading system for
assessing prognosis in patients with MDS.10 Multivariate analysis
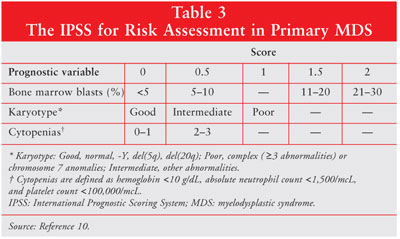
of 816 patients identified cytogenetic abnormalities, percentage of bone
marrow blasts, and the number of cytopenias as the most significant predictors
of survival and progression to AML (table 3).10 A person's total
score in the IPSS system is equal to the sum of the individual scores for bone
marrow blasts, karyotype, and cytopenias. Patients are then separated into
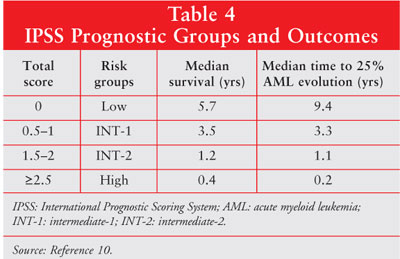
four risk groups (low, intermediate-1 [INT-1], intermediate-2 [INT-2], or
high) based on the total score (table 4). The higher the IPSS score, the worse
the prognosis.10 Interestingly, poorer survival times occurred in
patients 60 years and older in the low and INT-1 groups but did not differ
substantially in the higher risk groups (INT-2 and high).10
TREATMENT
The course of MDS
and response to therapy are influenced by disease stage, patient age, and
indiv idual prognostic factors. Therefore, treatment for MDS must be
personalized. In patients with low-risk disease, goals of therapy include
resolution of cyto penias, delayed progression to AML, and increased quality
of life.3 In patients with high-risk disease, the goal of therapy
is to eliminate the abnormal clone, thereby prolonging disease-free and
overall survival.3 Consequently, appropriate treatment options
range from supportive care with blood transfusions or colony-stimulating
factors to intensive therapy with chemotherapy or allogeneic stem cell
transplantation (alloSCT). Currently, alloSCT is the only curative therapy for
MDS.11
The National Comprehensive
Cancer Network Practice Guidelines in Oncology for MDS use a patient's IPSS
risk category, age, and performance status to categorize treatment options.
Treatment options are separated into two groups: low-intensity therapy for
low- and INT-1-risk categories and high-intensity therapy for INT-2- and
high-risk categories.11 High-intensity therapies, which consist of
chemotherapy and alloSCT, are beyond the scope of this review and are
discussed extensively in several articles.12-14 Also, as uniform
definitions of response criteria do not exist, not all definitions for
response are included; these can be found in the original articles. A list of
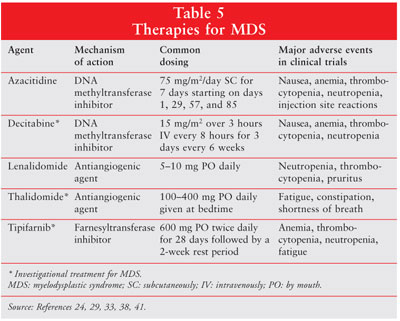
therapies used for the treatment of MDS can be found in Table 5.
Supportive Care
Supportive care
is currently the standard of care for the treatment of MDS and may be
clinically appropriate for patients with any IPSS score.11 Patients
are monitored for cytopenias and their associated adverse effects and are
treated with packed red blood cell (PRBC) transfusions for symptomatic anemia,
platelet transfusions for severe thrombocytopenia or bleeding, or antibiotics
for infections.
Hematopoietic cytokines can
be considered for refractory symptomatic anemias.11 Epoetin alfa
(Procrit/Amgen) has demonstrated effectiveness in treating anemia in patients
with MDS. In an open-label, multicenter, compassionate treatment trial, 100
patients received subcutaneous (SC) epoetin alfa 150 units/kg three times
weekly for a minimum of four weeks. This dose could be increased to 300
units/kg three times weekly if patients had no response to the lower dose. At
the conclusion of the study, 10 patients (10%) were deemed to have shown
response to hematocrit (Hct) (increase in Hct of 6% from baseline with no
transfusions for one month). Eighteen patients (18%) were considered to have
shown response to transfusion (50% decrease in transfusion requirement during
the final 12 weeks of the study).Epoetin alfa therapy was generally well
tolerated.15
Epoetin alfa in combination
with granulocyte colony-stimulating factor (G-CSF, filgrastim, Neupogen/
Amgen) has also been studied in the MDS population. A randomized phase II
trial evaluated the use of daily SC epoetin beta and filgrastim in patients
with RA, RARS, or RAEB according to the FAB classification. Patients were
randomized to one of two groups. The first group received filgrastim followed
by the epoetin beta/filgrastim combination; the second group received epoetin
beta followed by the epoetin beta/filgrastim combination. A total of 56
patients were included in the study, with 28 patients randomized to each
group. Complete erythroid response was defined as an increase in hemoglobin
(Hgb) to at least 11.5 g/dL. Partial erythroid response was defined as in
increase in Hgb by 1.5 g/dL in nontransfused anemia and a 100% reduction of
transfusion need in combination with stable Hgb for four weeks or more in
patients with pretreatment transfusion needs. Eighteen of the 47 evaluated
patients (38%) had an erythroid response (CR + PR) to treatment, and 10
patients (21%) demonstrated a complete response. There were no significant
differences, including response rates, between the two groups. Reported
adverse events were mild and included flu-like symptoms and local injection
site irritation.16
Other studies using various
forms of epoetin (alfa or beta) and dosing schedules with filgrastim have
shown similar improvements in erythroid response.17-19 Epoetin alfa
in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF,
sargramostim, Leukine/Berlex) has also been studied in the MDS population,
although response rates are generally lower.20 The use of G-CSF and
GM-CSF alone for the treatment of MDS has resulted in improved neutropenia
rates; however, data showing reduced infectious episodes, survival
prolongation, or reduced risk of transforming to AML do not exist for these
agents.21 Interleukin-11 (Oprelvekin, Neumega/Wyeth) has also been
studied in a small number of patients with MDS, although the patients could
concomitantly receive epoetin alfa for treatment of anemia and/or G-CSF for
treatment of neutropenia during the study.22 In addition,
darbepoetin alfa (Aranesp/Amgen) has produced 45% erythroid response rates in
a recent study of 48 patients with MDS.23
Low-Intensity Therapies
Azacitidine:
Azacitidine (Vidaza/ Pharmion) exerts its antineoplastic effects by causing
hypometh yl ation of DNA and direct cytotoxicity on abnormal hema topoietic
cells in the bone marrow. It is thought that hypo methylation may restore
normal function to genes needed for differentiation and proliferation.24
Azacitidine is incorporated into DNA where it shows dose- and time-dependent
inhibition of methyltransferase activity.25 Azacitidine is rapidly
absorbed after SC administration; approximately 89% of the dose is absorbed
based on area under the curve. Mean half-life after SC administration is 41
+/- 8 minutes. Urinary excretion is the primary method of elimination.24
Azacitidine is approved for
patients with MDS of all five FAB subtypes--RA or RARS (if accompanied by
neutropenia or thrombocytopenia or requiring transfusions), RAEB, RAEB-t, and
CMML. The recommended starting dose is 75 mg/m2 SC daily for seven
days every four weeks. The dose may be increased to 100 mg/m2 if no
beneficial effects are seen after two treatment cycles and if the patient
experiences no toxicity other than nausea and vomiting. Other dosing
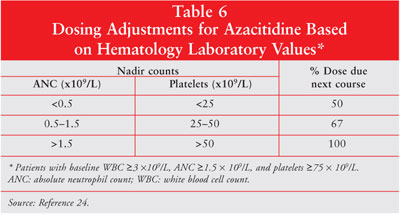
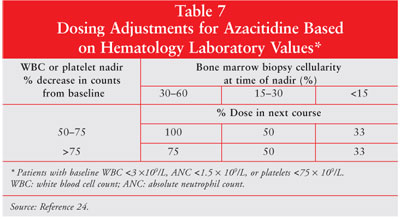
adjustments are required based on hematology laboratory values (tables 6, 7).
24 Azacitidine dose should be reduced by 50% if patients experience an
unexplained reduction in serum bicarbonate level of less than 20 mEq/L.
Similarly, if the blood urea nitrogen or serum creatinine levels become
elevated, the dose of azacitidine should be held until the values return to
normal or baseline. The subsequent dose should be reduced by 50% for the next
treatment course.24
A pivotal phase III study of the
Cancer and Leukemia Group B (CALGB) compared supportive care and SC
administration of azacitidine in patients meeting the FAB classification of
MDS. Patients with RA or RARS were required to show additional signs of
significant marrow dysfunction, such as symptomatic anemia requiring PRBC
transfusions for at least three months before study entry, thrombocytopenia
with two or more platelet counts <=50 ¥ 109/L or a
significant hemorrhage requiring platelet transfusions, or neutropenia with an
absolute neutrophil count (ANC) <1 ¥ 109/L and an infection
requiring intravenous antibiotics.26
This randomized open-label
study enrolled 191 patients, with 99 patients receiving azacitidine and 92
patients receiving supportive care. Azacitidine 75 mg/m2/day was
administered SC in seven-day cycles beginning on days 1, 29, 57, and 85. The
azacitidine dose could be increased to 100 mg/m2/day according to
previously stated criteria. Patients were assessed after the fourth cycle.
Definitions of response criteria are included in table 8.26
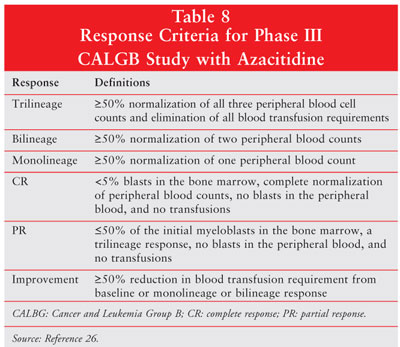
In the azacitidine group, 60% of
patients showed response (P<.0001), 7% had a complete response, 16% showed
a partial response, and 37% demonstrated improvement. Of the 92 patients
randomized to supportive care, none had a complete or partial response and 5%
met the criteria for improvement. Trilineage response was 23% for azacitidine
and 0% for supportive care. The median time to AML transformation or death was
21 months in patients who received azacitidine and 12 months in patients who
received supportive care (P=.007). Forty-nine patients crossed over from the
supportive care group; 47% of those patients showed response, 10% had a
complete response, 4% showed a partial response, and 33% demonstrated
improvement. The median survival was 20 months in the azacitidine group,
compared to 14 months in the supportive care group (P=.10). To reduce the
confounding data regarding the crossover patients, a second analysis was
performed comparing patients in the azacitidine group to those in the
supportive care group who did not cross over or who crossed over late in the
study (after six months). In this analysis, azacitidine had improved median
survival, compared to the supportive care subgroup (P=.03). The most common
toxicity of azacitidine was myelosuppression. Grade 3 or 4 neutropenia
occurred in 59%, granulocytopenia in 81%, and thrombocytopenia in 70% of
patients who received azacitidine.26 Other common adverse events of
azacitidine that have been reported in clinical trials include nausea,
vomiting, pyrexia, diarrhea, constipation, injection site erythema, and
ecchymosis.24
In a separate evaluation of
the previous study, quality-of-life assessments were analyzed. Patients in the
azacitidine treatment group experienced greater improvement in fatigue (P
=.001), dyspnea (P=.0014), physical functioning (P=.0002), psychological
distress (P=.015), and positive affect (P=.0077), compared with patients in
the supportive care group.27
Lenalidomide:
Lenalidomide (Revlimid/Celgene Corp.) is an immunomodulatory agent with a
mechanism of action similar to thalidomide.28 An open-label,
single-center trial evaluated the use of lenalidomide in 43 patients with MDS
who had symptomatic anemia. Patients were randomized to receive lenalidomide
25 mg daily continuously, 10 mg daily continuously, or 10 mg daily for 21 days
in every 28-day cycle. All treatment groups received lenalidomide orally.
Sequential dose reductions were permitted. Patients included in the study had
a diagnosis of MDS based on the FAB criteria for greater than three months and
either symptomatic anemia (Hgb<10 g/dL) or transfusion dependence (>=4
units PRBC in previous eight weeks). Patients also had no response to either
recombinant erythropoietin or an endogenous erythropoietin level of >500
mU/mL. Overall, 24 patients (56%) had a hematologic response. In 32 patients
who were previously transfusion dependent, 20 achieved transfusion
independence. After 81 weeks of evaluation, the median duration of a major
response had not been reached. Of the 10 patients who achieved a complete
cytogenetic response (absence of pretreatment cytogenetic abnormalities), nine
had the
Due to the results of this
study and an abstract presented at the annual American Society of Clinical
Oncology (ASCO) meeting in 2005, the FDA approved lenalidomide for the
treatment of transfusion-dependent anemia due to low- or INT-1-risk MDS
associated with a
Investigational Therapies
DNA
Methyltransferase Inhibitors: The cytosine analog decitabine
(Dacogen/MGI Pharma), originally synthesized in the 1960s, is an analog of
azacitidine capable of inhibiting DNA methyltransferase.32,33 The
results of a randomized, open-label, phase III trial enrolling 170 patients
were recently reported at the 2005 annual ASCO meeting. The study compared
decitabine (n=89) plus supportive care to supportive care alone (n=81) in
patients with IPSS INT-1 (31%), INT-2 (44%), and high-risk (26%) MDS.
Decitabine was administered as a three-hour infusion of 15 mg/m2
every eight hours for three consecutive days every six weeks. Response rates
were 17% for decitabine, compared to 0% for supportive care alone (P<.001).
Of the 15 patients (17%) who achieved a response, eight (9%) had complete
responses and seven (8%) had partial responses. In addition, hematologic
improvements were observed in an additional 13% of patients who received
decitabine and 7% of patients who received supportive care. The probability of
progression to AML or death was 1.72-fold greater in the supportive care group
than in the decitabine group (P=.017). Median time to progression to AML or
death was 340 days in patients receiving decitabine, compared to 219 days in
patients receiving supportive care (P=.043). Myelosuppression was the most
common toxicity in the decitabine group, and febrile neutropenia was the most
common grade 3 or 4 toxicity (most serious toxicity, range 0 to 4).
34 Additional data in this study are limited due to the abstract format
of the report. These results are the basis of an FDA submission, currently
under review for the treatment of MDS.35
Antiangiogenic
Therapies: Thalidomide (Thalomid/Celgene Corp.) is probably most
widely known for its teratogenicity, which caused a variety of deformities in
infants, specifically, amelia (lack of limb) and phocomelia (seal limb).36
Thalidomide is a potent inhibitor of vascular endothelial growth factor and
basic fibroblast growth factor, which are both needed for angiogenesis. These
angiogenic factors as well as others have been identified in the bone marrow,
plasma, and blood cells of patients with MDS.37 Four phase II
studies have evaluated thalidomide as single-agent therapy in MDS.
In the largest phase II
study to date, patients with MDS of all morphological subtypes received
thalidomide at doses ranging from 100 to 400 mg/day given at bedtime. A total
of 83 patients were enrolled, with 32 patients discontinuing thalidomide
before 12 weeks of treatment. Of these 32 patients, one patient never started
thalidomide, six had disease progression, 11 had other medical problems, and
14 discontinued therapy due to side effects. At study end, there were no
patients with a complete response. Of the 16 patients with hematologic
improvement, 15 patients had an erythroid response and one patient had a
platelet response. The most common side effects in this study were fatigue
(79%), constipation (71%), and shortness of breath (54%). However, fewer than
5% of patients experienced grade 4 toxicity.38
The major benefit of
thalidomide in the treatment of patients with MDS is transfusion independence.
Further studies are needed to assess the durability of hematologic response
and impact on quality of life.
Farnesyltransferase
Inhibitors: The Ras system of proteins is a cellular signaling pathway
that controls cell growth, proliferation, and cell death. Experimental studies
have shown that mutated forms of Ras have been found in a wide range of
malignancies, including MDS.39,40 A key process in the Ras pathway
is prenylation, which is carried out by one of two enzymes--farnesyltransferase
or geranylgeranyltransferase.39 The most extensively studied
farnesyltransferase inhibitor in MDS is tipifarnib (Zarnestra/Johnson &
Johnson). A phase II study evaluated the use of tipifarnib in patients with
MDS with intermediate- (INT-1, INT-2) or high-risk IPSS scores. Patients were
treated with tipifarnib at a starting dose of 600 mg by mouth twice daily for
28 days followed by a two-week rest period (six weeks = one course). Of the 28
patients enrolled, three patients showed response, with two having a complete
response and one demonstrating a partial response. The most common side effect
was myelosuppression, with 79% of patients experiencing anemia, 75%
experiencing thrombo cytopenia, and 61% experiencing neutropenia.41
Additionally, 11% and 21% of patients receiving tipifarnib experienced
neurotoxicity and rash, respectively.41 In a second phase II study
presented at the annual meeting of the American Society of Hematology, 82
patients with MDS were treated with tipifarnib, resulting in responses seen in
28 patients (33%).42
Recently, the FDA's
Oncologic Drugs Advisory Committee rejected an accelerated approval request
for tipifarnib in patients with AML.43 Studies with tipifarnib in
patients with MDS are ongoing.
In addition to the
previously mentioned agents, other novel therapies are undergoing clinical
trials. Arsenic trioxide is currently under evaluation in phase II clinical
trials in patients with high- and low-risk MDS. Lonafarnib, a
farnesyltransferase inhibitor, has undergone phase II trials in patients with
advanced MDS and CMML. Small-molecule inhibitors of the VEGF receptor tyrosine
kinases SU5416 and SU11248 have produced dose-limiting nonhematologic toxicity
in some cases and low response rates. Bevacizumab is currently undergoing
phase II trials in MDS. Other agents of interest include bortezomib
(proteasome inhibitor), TLK199 (liposomal glutathione derivative), and
antisense oligonucleotides to inactivated p53 RNA.21
Conclusion
MDS is a
collection of disorders that is difficult to manage clinically. The advanced
age of patients at diagnosis makes administration of cytotoxic chemotherapy or
alloSCT challenging. Furthermore, with the exception of alloSCT, there are no
curative therapies available for treatment of MDS, and supportive care
continues to remain the standard of care. Currently, azacitidine and
lenalidomide are the only FDA-approved treatments for MDS, although several
other therapies are either in clinical trials or the approval process. Some of
these investigational therapies currently in clinical studies appear
promising, and patients with this uncommon disorder stand to benefit from the
availability of additional agents.
REFERENCES
1. Heaney ML,
Golde DW. Myelodysplasia. N Engl J Med. 1999;340:1649-1660.
2. Leone G, Mele L,
Pulsoni A, et al. The incidence of secondary leukemias. Haematologica.
1999;84:937-945.
3. Rowe JM. State of
the science for myelodysplastic syndrome: prognosis and promise of new
therapies. Best Pract Res Clin Haematol. 2004;17:535-541.
4. Aul C, Germing U,
Gattermann N, Minning H. Increasing incidence of myelodysplastic syndromes:
real or fictitious? Leuk Res. 1998;22:93-100.
5. Mufti GJ.
Pathobiology, classification, and diagnosis of myelodysplastic syndrome.
Best Pract Res Clin Haematol. 2004;17:543-557.
6. Estey EH.
Prognosis and therapy of secondary myelodysplastic syndromes. Haematologica
. 1998;83:543-549.
7. Faderl S,
Kantarjian HM. Novel therapies for myelodysplastic syndromes. Cancer.
2004;101:226-241.
8. Bennett JM,
Catovsky D, Daniel MT, et al. Proposals for the classification of the
myelodysplastic syndromes. Br J Haematol. 1982;51:189-199.
9. Vardiman JW,
Harris NL, Brunning RD. The World Health Organization (WHO) classification of
the myeloid neoplasms. Blood. 2002;100:2292-2302.
10. Greenberg P, Cox
C, LeBeau MM, et al. International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood. 1997;89:2079-2088.
11. National
Comprehensive Cancer Network. Practice Guidelines in Oncology: Myelodysplastic
Syndromes. Version 1. 2005. Available at: www.nccn.org/
professionals/physician_gls/PDF/mds.pdf. Accessed
12. Foss FM.
Nucleoside analogs and antimetabolite therapies for myelodysplastic syndrome.
Best Pract Res Clin Haematol. 2004;17:573-584.
13. Giralt S. Bone
marrow transplant in myelodysplastic syndromes: new technologies, same
questions. Curr Hematol Rep. 2005;4:200-207.
14. McCarty J.
Transplant strategies for myelodysplastic syndrome. Best Pract Res Clin
Haematol. 2004;17:559-572.
15. Rose EH, Abels
RI, Nelson RA, et al. The use of r-HuEpo in the treatment of anaemia related
to myelodysplasia (MDS). Br J Haematol. 1995;89:831-837.
16.
Hellstrom-Lindberg E, Ahlgren T, Beguin Y, et al. Treatment of anemia in
myelodysplastic syndromes with granulocyte colony-stimulating factor plus
erythropoietin: results from a randomized phase II study and long-term
follow-up of 71 patients. Blood. 1998;92:68-75.
17. Negrin RS, Stein
R, Vardiman J, et al. Treatment of the anemia of myelodysplastic syndromes
using recombinant human granulocyte colony-stimulating factor in combination
with erythropoietin. Blood. 1993;82:737-743.
18. Mantovani L,
Lentini G, Hentschel B, et al. Treatment of anaemia in myelodysplastic
syndromes with prolonged administration of recombinant human granulocyte
colony-stimulating factor and erythropoietin. Br J Haematol.
2000;109:367-375.
19. Casadevall N,
Durieux P, Dubois S, et al. Health, economic, and quality-of-life effects of
erythropoietin and granulocyte colony-stimulating factor for the treatment of
myelodysplastic syndromes: a randomized, controlled trial. Blood.
2004;104:321-327.
20. Thompson JA,
Gilliland DG, Prchal JT, et al. Effect of recombinant human erythropoietin
combined with granulocyte/macrophage colony-stimulating factor in the
treatment of patients with myelodysplastic syndrome. Blood.
2000;95:1175-1179.
21. Jabbour EJ, Giles
FJ. New agents in myelodysplastic syndromes. Curr Hematol Rep.
2005;4:191-199.
22. Tsimberidou AM,
Giles FJ, Khouri I, et al. Low-dose interleukin-11 in patients with bone
marrow failure: update of the M.D Anderson Cancer
23. Stasi R,
Abruzzese E, Lanzetta G, et al. Darbepoetin alfa for the treatment of anemic
patients with low- and intermediate-1-risk myelodysplastic syndromes. Ann
Oncol. 2005:16;1921-1927.
24. Pharmion
Corporation. Vidaza package insert. Boulder, CO. August 2004. Available at:
www.vidaza.com/corporateweb/vidazaus/homeb.nsf/AttachmentsBy
Title/Packaging/$FILE/FullPrescribingInformationForVidaza.pdf.
25. Silverman LR. DNA
methyltransferase inhibitors in myelodysplastic syndrome. Best Pract Res
Clin Haematol. 2004;17:585-594.
26. Silverman LR,
Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in
patients with the myelodysplastic syndrome: a study of the cancer and leukemia
group B. J Clin Oncol. 2002;20:2429-2440.
27. Kornblith AB,
Herndon JE 2nd, Silverman LR, et al. Impact of azacytidine on the quality of
life of patients with myelodysplastic syndrome treated in a randomized phase
III trial: a Cancer and Leukemia Group B study. J Clin Oncol.
2002;20:2441-2452.
28. Sekeres MA, List
A. Lenalidomide (Revlimid, CC-5013) in myelodysplastic syndromes: is it any
good? Curr Hematol Rep. 2005;4:182-185.
29. List A, Kurtin S,
Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N
Engl J Med. 2005;352:549-557.
30. List AF, Dewald
G, Bennett J, et al. Hematologic and Cytogenetic (CTG) Response to
Lenalidomide (CC-5013) in Patients with Transfusion-Dependent (TD)
Myelodysplastic Syndrome (MDS) and Chromosome 5q31.1 Deletion: Results of the
Multicenter MDS-003 Study. Poster presented at 41st Annual American Society of
Clinical Oncology Meeting.
31. Celgene
Corporation. Revlimid package insert.
32. Issa JP.
Decitabine. Curr Opin Oncol. 2003;15:446-451.
33.
34. Saba HI,
Rosenfeld CS, Issa J, et al. Clinical benefit and survival endpoints from a
phase III trial comparing decitabine (DAC) vs. supportive care (SC) in
patients with advanced myelodysplastic syndromes (MDS). Poster presented at
41st Annual American Society of Clinical Oncology Meeting.
35. SuperGen, Inc.
Web site. Dacogen NDA Accepted for Filing by FDA. Available at:
ir.supergen.com/phoenix.zhtml?c=105560&p=irol-newsArticle&ID=658150&
highlight=. Accessed
36. Matthews SJ,
McCoy C. Thalidomide: a review of approved and investigational uses. Clin
Ther. 2003;25:342-395.
37. Estey EH.
Modulation of angiogenesis in patients with myelodysplastic syndrome. Best
Pract Res Clin Haematol. 2004;17:623-639.
38. Raza A, Meyer P,
Dutt D, et al. Thalidomide produces transfusion independence in long-standing
refractory anemias of patients with myelodysplastic syndromes. Blood.
2001;98:958-965.
39. Feldman EJ.
Farnesyltransferase inhibitors in myelodysplastic syndrome. Curr Hematol Rep
. 2005;4:186-190.
40. Boguski MS,
McCormick F. Proteins regulating Ras and its relatives. Nature.
1993;366:643-654.
41. Kurzrock R,
Albitar M, Cortes JE, et al. Phase II study of R115777, a farnesyl transferase
inhibitor, in myelodysplastic syndrome. J Clin Oncol. 2004;22:1287-1292.
42. Kurzrock R,
Fenaux P, Raza A, et al. High-Risk Myelodysplastic Syndrome (MDS): First
results of international phase 2 study with oral farnesyltransferase inhibitor
R115777 (Zarnestra). Presented at the 46th Annual Meeting of the American
Society of Hematology.
43. FDA Advisory
Committee Web site. Zarnestra Phase III Data Needed Pre-Approval, Oncology
Committee Says. Available at: www.fdaadvisorycommittee.
com/FDC/AdvisoryCommittee/Committees/Oncologic+Drugs/050505_
zarnestra/050505_ZarnestraR.htm. Accessed
To comment on this article,
contact
editor@uspharmacist.com.






