US Pharm. 2011;36(1)(Oncology suppl):7-9.
ABSTRACT: Precise data are not available, but the occurrence of drug-drug interactions in oncology is theorized to be higher than in most other conditions. The three primary types of drug interactions are pharmaceutical, pharmacodynamic, and pharmacokinetic. Metabolic interactions are the most common, but other types of interactions can occur in patients undergoing chemotherapy. It is important for pharmacists to competently advise patients about what to expect and to make appropriate recommendations to other health care professionals.
With the vast array of drug interactions that are possible with chemotherapy agents, it is essential that pharmacists be confident about advising patients on what to expect and about making recommendations to other health care professionals. Typically, cancer patients take a number of medications to manage related conditions, such as pain and nausea. While a discussion of every possible interaction is beyond the scope of this article, an attempt is made to explain the mechanisms of the different types of interactions.
Introduction
A drug-drug interaction (DDI) occurs when the clinical effect of a given drug is altered by the action of another drug.1 Importantly, drug interactions contribute to the majority of adverse drug reactions, and approximately 70% of interactions are clinically relevant.2 While exact data on the occurrence of DDIs in oncology are not available, the occurrence is theorized to be higher than in most other conditions since many cancer patients take an array of medications for comorbid conditions such as pain, depression, and seizures.1,3 The risk of DDIs is increased by the patient's age (about 60% of cancer patients are aged over 65 years) and pharmacogenetic makeup and by the presence of malnutrition, malabsorption, or impaired renal or hepatic function.3,4 Furthermore, oncology drugs generally have a narrow therapeutic index, making any DDI highly significant.4 A comprehensive knowledge of drug interactions, patient education, intervention, and drug monitoring will reduce the risk of adverse effects, increase the efficacy of the regimen, and minimize treatment costs.5,6
There are three main types of drug interactions: pharmaceutical, pharmacodynamic, and pharmacokinetic (see TABLE 1). As will be explained below, not all drug interactions are negative.
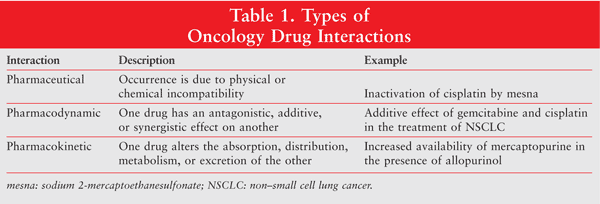
Pharmaceutical Interactions
Pharmaceutical interactions occur because of a physical or chemical incompatibility. A classic example of an unwanted pharmaceutical interaction is the inactivation of the platinum compound cisplatin by the addition of the thiol mesna (sodium 2-mercaptoethanesulfonate). If these compounds are combined for infusion, a mesna-platinum adduct forms.7 Similarly, when dissolved in 5% dextrose infusion fluid, mitomycin C rapidly degrades into inactive mitosenes.7 Taxanes, epipodophyllotoxins, and 5-fluorouracil (5-FU) have been shown to precipitate in infusion fluids.7
Positive interactions may be used to optimize drug therapy. For example, to modulate the pharmacokinetic and pharmacodynamic properties of doxorubicin, the drug is encapsulated in pegylated liposomes. This results in a pharmaceutical interaction that reduces the cardiotoxicity associated with doxorubicin.7 A similar interaction occurs between cisplatin and Cremophor EL, the vehicle used in paclitaxel. Cremophor EL has been shown to selectively inhibit the accumulation of cisplatin in peripheral blood leukocytes; however, it has no effect on cisplatin accumulation in tumor cells. This finding has led to the conclusion that formulating cisplatin with Cremophor EL has the potential to improve cisplatin's therapeutic index and reduce its dosing frequency.8
Pharmacodynamic Interactions
Pharmacodynamic interactions are those in which one drug has an antagonistic, additive, or synergistic effect on another. Such interactions are of particular importance when chemotherapy drugs that are nephrotoxic are combined. An additive effect on the kidneys can lead to mild-to-moderate renal failure.9 On the other hand, synergistic activity allows for lower dosing of the drugs used in combination. This is potentially beneficial, as the patient experiences fewer side effects with each drug.
Gemcitabine has been studied in combination with a number of other chemotherapeutic agents for its synergistic effect in the treatment of non-small cell lung cancer (NSCLC).10 Gemcitabine is an ideal drug for combination chemotherapy because it has not shown any toxicity when combined with other agents. Furthermore, its mode of action differs from other chemotherapy regimens used for NSCLC.10 In particular, the combinations of gemcitabine with cisplatin, gemcitabine with vindesine, and gemcitabine with pemetrexed have shown promising results.10,11 Nucleotide-pool modulation, drug metabolism, and cellular DNA repair capacity contribute to this synergistic effect.
The camptothecin analogue topotecan has synergistic activity with cisplatin, doxorubicin, etoposide, paclitaxel, and cytarabine, in decreasing order of occurrence.12 Of particular interest, therefore, are the combinations of cisplatin with topotecan and cisplatin with doxorubicin.12 It is proposed that the inhibition of topoisomerase I by topotecan may alter cells' ability to repair cisplatin adducts.13
In a synergistic reaction, leucovorin enhances the cytotoxicity of 5-FU, an effect that is greater than that occurring with the combination of methotrexate and 5-FU. This combination frequently is used in the management of colon cancer.14
Since thalidomide potentiates the sedative effects of benzodiazepines, opiates, hypnotics, and alcohol, patients should be warned about increased drowsiness when these drugs are taken in combination. Additionally, patients taking thalidomide who are also receiving dexamethasone or doxorubicin should be given warfarin or low-molecular-weight heparins, since this combination increases the risk of venous thromboembolism.15 A similar effect is noted for lenalidomide.15
Pharmacokinetic Interactions
A pharmacokinetic interaction occurs when one medication alters the absorption, distribution, metabolism, or excretion of another. The most common pharmacokinetic interactions noted in oncology are those involving the modulation of hepatic metabolism by CYP450.
The effect of other drugs or nonmedicinal products on the amount of chemotherapeutic drug absorbed is especially important in the case of orally administered drugs. Food intake may increase or decrease these drugs' absorption. For example, it is important that capecitabine be taken within 30 minutes after a meal. The safety of capecitabine taken on an empty stomach has not been established.16 When pharmacists dispense prescriptions for these agents, it is essential that they make patients aware of the effect or food or drugs.
The enzyme xanthine oxidase inactivates mercaptopurine into thiouric acid. Since allopurinol inhibits xanthine oxidase, combined use slows the breakdown of mercaptopurine. This causes an increase in the mercaptopurine available for anabolic conversions that produce cytotoxic products such as thioguanine. It is recommended that the dose of mercaptopurine be reduced to 25% to 33% of the normal dose in patients requiring both drugs. A similar interaction is seen with azathioprine and allopurinol.17
Additionally, since most antineoplastic agents affect the gastrointestinal mucosa, they have the potential to interfere with the absorption of other orally administered drugs.18
The major factor affecting the distribution of drugs is how a drug binds to proteins such as albumin, alpha1-acid glycoprotein, lipoproteins, immunoglobulins, and erythrocytes. Drugs, such as phenytoin, that are highly bound to plasma proteins may be displaced by agents that have a higher affinity for the protein, leading to an increased concentration of the active drug.8 Drugs such as paclitaxel and etoposide are highly protein bound; therefore, they interact with other protein-bound drugs (e.g., warfarin).7 A similar effect may be seen with drugs that are transported by cell-bound transport proteins such as P-glycoprotein.9
Most antineoplastic drugs are excreted by hepatic metabolism into byproducts that are less lipid soluble. Metabolism may involve either a phase I reaction--in which products undergo oxidation, reduction, or hydrolysis--or a phase II reaction, in which products undergo conjugation. The CYP450 system is a group of heme protein mono-oxygenase enzymes that are largely responsible for phase I oxidative metabolism of drugs.9,19 The CYP450 enzymes may be modulated by many other drugs, particularly corticosteroids and anticonvulsants.7 Out of the 100 isoenzymes, CYP3A4, -2D6, -2C19, and -2C9 are the most prominent in terms of activity.9
Oxazaphosphorines such as cyclophosphamide and ifosfamide; taxanes such as paclitaxel and docetaxel; and lapatinib, sorafenib, nilotinib, dasatinib, imatinib, sunitinib, and erlotinib are all partly metabolized by CYP3A4. Combining these drugs with other CYP3A4 substrates, inducers, or inhibitors will affect their action. Cyclophosphamide is a reversible inhibitor of CYP3A4, so caution should be exercised when this drug is used with other CYP3A4 substrates, such as those listed in TABLE 2.15,20
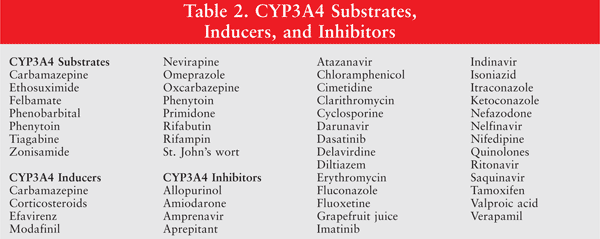
Tamoxifen, a selective estrogen receptor modulator, has either weak estrogenic or antiestrogenic activity, depending on the type of tissue in question. It is highly metabolized by the CYP450 system into active metabolites, the most active of which are endoxifen and 4-hydroxytamoxifen. Many studies have reported lower endoxifen concentrations in women with impaired CYP2D6 metabolism, and, consequently, a high rate of breast cancer recurrence. Based on this, drugs that interfere with CYP2D6 metabolism may have a similar effect in patients taking tamoxifen.21
CYP2D6 inhibitors have the potential to lessen tamoxifen's effectiveness. This includes several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors that may be used to treat depression associated with breast cancer. The most potent of these are fluoxetine, paroxetine, and duloxetine; weaker inhibitors include citalopram, escitalopram, fluvoxamine, and sertraline. Venlafaxine has little or no effect on the metabolism of tamoxifen.21,22 Gabapentin may be safely used for the treatment of hot flashes in women, as it is a weak inhibitor of CYP2D6. Aside from chlorpromazine, whose potency is similar to that of citalopram and sertraline, all antipsychotics are weak inhibitors of CYP2D6. Quinidine is the most potent CYP2D6 inhibitor in the cardiac drug class, followed by ticlodipine, amiodarone, and calcium channel blockers. In general, antihistamines are moderate inhibitors of CYP2D6.
Doxorubicin and vinblastine are also metabolized by CYP2D6 enzymes. The abovementioned drugs, therefore, will have a similar effect on doxorubicin and vinblastine.
Cancer patients receiving warfarin therapy should have their international normalized ratio (INR) monitored regularly and the dose of warfarin adjusted accordingly. Most interactions between chemotherapy agents and warfarin appear to be caused by inhibition of the CYP450 system. Patients taking gemcitabine have an increased INR and, consequently, a reduced requirement for warfarin. This may be due either to decreased warfarin metabolism as a result of CYP450 inhibition or to decreased synthesis of clotting factors.23 A similar effect is noted with 5-FU and capecitabine, as well as with tamoxifen.23 Furthermore, since both tamoxifen and warfarin are highly protein bound, a reaction that displaces warfarin from proteins and increases the concentration of free warfarin may contribute to this effect.24 Paclitaxel also has been shown to cause chemotherapy-induced protein displacement of warfarin, increasing the patient's INR.25
Most anticancer drugs are eliminated via metabolism, with the exception of platinum and methotrexate, which are excreted mainly by the kidneys through glomerular filtration and active tubular secretion. By competing for tubular secretion by the kidneys, probenecid, salicylates, and trimethoprim-sulfamethoxazole all increase plasma methotrexate concentrations to toxic levels.17,26 Similarly, cisplatin has been shown to increase the toxic effects of lithium and topotecan by altering their renal clearance.17
Conclusion
The importance of drug interactions in oncology cannot be stressed enough. A sound knowledge of potential interactions can greatly assist in reducing occurrence and minimizing treatment costs. While metabolic interactions are the most common type of interaction seen in oncology, pharmacists should bear in mind that other types of interactions may occur when patients are undergoing chemotherapy.
REFERENCES
1. Riechelmann RP, Del Giglio A. Drug interactions in oncology: how common are they? Ann Oncol. 2009;20:1907-1912.
2. Köhler GI, Bode-Böger SM, Busse R, et al. Drug-drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38:504-513.
3. Riechelmann RP, Saad ED. A systematic review on drug interactions in oncology. Cancer Invest. 2006;24:704-712.
4. Chan A, Tan S, Wong CM, et al. Clinically significant drug-drug interactions between oral anticancer agents and nonanticancer agents: a Delphi survey of oncology pharmacists. Clin Ther. 2009;31(theme issue):2379-2386.
5. Kuhlmann J, Mück W. Clinical-pharmacological strategies to assess drug interaction potential during drug development. Drug Saf. 2001;24:715-725.
6. Corcoran ME. Polypharmacy in the Older Patient With Cancer. Cancer Control. 1997;4:419-428.
7. Beijnen JH, Schellens JH. Drug interactions in oncology. Lancet Oncol. 2004;5:489-496.
8. Gelderblom H, Loos WJ, Verweij J, et al. Modulation of cisplatin pharmacodynamics by Cremophor EL: experimental and clinical studies. Eur J Cancer. 2002;38:205-213.
9. Haidar C, Jeha S. Drugs in childhood cancer. Lancet Oncol. [Epub ahead of print September 23, 2010.]
10. Kanzawa F, Saijo N. In vitro interaction between gemcitabine and other anticancer drugs using a novel three-dimensional model. Semin Oncol. 1997;24(2 suppl 7):S7-8-S7-16.
11. Giovannetti E, Danesi R, Mey V, et al. In vitro studies on gemcitabine combinations with other antiblastics. Ann Oncol. 2006;17(suppl 5):v17-v19.
12. Jonsson E, Fridborg H, Nygren P, Larsson R. Synergistic interactions of combinations of topotecan with standard drugs in primary cultures of human tumor cells from patients. Eur J Clin Pharmacol. 1998;54:509-514.
13. Romanelli S, Perego P, Pratesi G, et al. In vitro and in vivo interaction between cisplatin and topotecan in ovarian carcinoma systems. Cancer Chemother Pharmacol. 1998;41:385-390.
14. Ardalan B, Luis R, Jaime M, Franceschi D. Biomodulation of fluorouracil in colorectal cancer. Cancer Invest. 1998;16:237-251.
15. Lohr LK. Drug interactions with newer oral chemotherapy agents. US Pharm. 2009;34(7)(Oncology suppl):4-8.
16. Tyler T. Drug interactions in metastatic breast cancer. J Oncol Pharm Pract. [Epub ahead of print September 22, 2010.]
17. Balis FM. Pharmacokinetic drug interactions of commonly used anticancer drugs. Clin Pharmacokinet. 1986;11:223-235.
18. Sweetman SC, ed. Martindale: The Complete Drug Reference. 34th ed. London, England: Pharmaceutical Press; 2005 [electronic version].
19. Le Blanc GA, Waxman D. Interactions of anticancer drugs with hepatic monooxygenase enzymes. Drug Metab Rev. 1989;20:395-439.
20. Yap KY, Chui WK, Chan A. Drug interactions between chemotherapeutic regimens and antiepileptics. Clin Ther. 2008;30:1385-1407.
21. Sideras K, Ingle JN, Ames MM, et al. Coprescription of tamoxifen and medications that inhibit CYP2D6. J Clin Oncol. 2010;28:2768-2776.
22. Henry NL, Stearns V, Flockhart DA, et al. Drug interactions and pharmacogenomics in the treatment of breast cancer and depression. Am J Psychiatry. 2008;165:1251-1255.
23. Saif MW, Wasif N. Interaction between capecitabine and gemcitabine with warfarin in a patient with pancreatic cancer. JOP. 2008;9:739-743.
24. Givens CB, Bullock LN, Franks AS. Safety of concomitant tamoxifen and warfarin. Ann Pharmacother. 2009;43:1867-1871.
25. Thompson ME, Highley MS. Interaction between paclitaxel and warfarin. Ann Oncol. 2003;14:500.
26. Voll ML, Yap KD, Terpstra WE, Crul M. Potential drug-drug interactions between anti-cancer agents and community pharmacy dispensed drugs. Pharm World Sci. 2010;32:575-580.
To comment on this article, contact rdavidson@uspharmacist.com.





