US Pharm. 2022;47(12):HS7-HS12.
ABSTRACT: Chylothorax, which is the accumulation of lymphatic fluid, or chyle, in the pleural space, occurs most commonly after thoracic surgery. Although it is rare, chylothorax has devastating consequences if not recognized early and managed appropriately. The standard diagnostic approach is the identification of triglycerides and chylomicrons in the pleural fluid. Conservative management consists of reducing chyle flow via complete elimination of fat intake or lessening chyle production with use of octreotide or somatostatin. Surgical treatment is indicated based on lack of response to conservative management. Interventional management techniques are currently evolving. An understanding of the pathophysiology of the chyle leak will inform the diagnosis and management of chylothorax. Overall, conservative management of this serious condition is quite successful.
Chylothorax (also known as chylous effusion) is the accumulation of lymphatic fluid, or chyle, in the pleural space due to an obstruction or disruption of the thoracic duct or a major lymphatic tributary. This rare but serious condition, which manifests as fluid buildup between the tissues lining the lungs and chest (pleural effusion), most commonly occurs as a complication of thoracic surgery. Chylothorax can lead to significant morbidity and mortality. Historically, the rate of mortality was approximately 50%, but heightened vigilance as well as improvements in treatment options have reduced the mortality rate.1-5 This article will describe the clinical characteristics, etiologies, and treatment strategies for chylothorax and provide a brief review of the relevant literature.
ANATOMY AND PHYSIOLOGY
The thoracic duct, which is 36 cm to 45 cm long and has a diameter of 2 mm to 3 mm, is the largest collecting channel of the lymphatic system. It originates in the cisterna chyli, a lymphatic sac located anterior to the second lumbar vertebra and posterolateral to the abdominal aorta. The thoracic duct enters the thorax through the aortic habitus and ascends extrapleurally along the right anterior surface of the vertebral bodies; then it crosses to the left side of the mediastinum and ascends extrapleurally on the left side of the esophagus at the level of the fifth to seventh thoracic vertebrae. At the base of the neck, the thoracic duct turns caudally, entering the venous system at the subclavian-internal jugular vein junction on the left. In 40% of the population, the thoracic duct divides into two or more branches. These branches may form a complex in the mid portion of the duct and may end up independent or as one duct. Occasionally, the upper portion of the duct divides into two branches that drain separately, one in the usual fashion and the other reaching the right subclavian vein. This anatomical variation describes the development of thoracic duct injury or chyle leak in trauma involving the esophagus, the thoracic spine, and the aortic and subclavian area.1,3,5,6
The thoracic duct is responsible for transporting chyle from the intestines and lymphatic fluid from the peritoneum, abdominal wall, and lower extremities to the venous system. Each day, the thoracic duct empties 1,500 mL to 2,500 mL of chyle into the venous system. The volume varies from 10 mL/kg to 100 mL/kg body weight per day, depending on the dietary-fat content, bowel function, and degree of activity. The volume increases postprandially on a fat-rich diet and decreases during periods of immobility, starvation, or gastric suction, wherein the flow may be as low as 10 mL/h to 15 mL/h.1,6-8
Chyle contains fat (especially long-chain fatty acids), cholesterol, triglycerides, chylomicrons, fat-soluble vitamins, and lymph. It also contains immunoglobulins, enzymes, and WBCs (most of them lymphocytes of the T-cell variety), making it bacteriostatic. Consequently, infections are rare in this type of effusion. Chylous effusion is odorless, and the high triglyceride content and chylomicrons give it a milky appearance. The effusion may also possess a milky appearance in pseudochylothorax or in empyema. Pseudochylothorax likely results from long-standing pleural effusion that mimics the appearance of chylothorax but lacks the chylomicrons that confirm a definitive diagnosis. The presence of pleural effusion with the aforementioned characteristics and etiologic factors denotes the possibility of chylothorax.1,6-8
ETIOLOGY
Chylothorax can be classified as traumatic (~50% of cases), nontraumatic (39%-72%), spontaneous or idiopathic (up to 6%), or congenital (rare).5,7-10 Traumatic chylothorax is further subclassified as iatrogenic (surgical) or noniatrogenic (nonsurgical). Thoracic surgery involving the heart, aorta, esophagus, and subclavian vessels is the most common cause of iatrogenic chylothorax.11-29 Noniatrogenic traumatic causes include penetrating trauma from a stab or gunshot wound, blunt thoracic trauma, thoracic spine fractures, stretching, violent coughing and sneezing, and seat belt use.9,10 Nontraumatic chylothorax arises from obstruction of lymphatic outflow, increased lymph production (i.e., liver cirrhosis, portal hypertension), and changes in lymph composition.9,30 The most common cause of nontraumatic chylothorax is malignancy (especially lymphoma) and multiple types of hematologic and solid tumors.31-34 Spontaneous chylothorax has no identifiable cause, but it may be associated with fixation of the thoracic duct in previous trauma. Congenital chylothorax, the most common form of pleural effusion occurring in newborns, results from damage to the thoracic duct after cardiothoracic surgery, birth trauma, or great vessel thrombosis. Other unusual occurrences of spontaneous chylothorax include amyloidosis/sarcoidosis, superior vena cava syndrome and thoracic aortic aneurysm, tuberculosis, Behçet disease, and Gorham-Stout disease.30,35-38
DIAGNOSIS AND CLINICAL PRESENTATION
Once chylothorax is suspected, pleural fluid, laboratory analysis, and imaging should be considered in order to establish the diagnosis. The fluid may not always look milky; for example, it may be stained with blood due to trauma or be serous if the patient is fasting. Measuring triglycerides in the pleural fluid will further assist in diagnosis; a level of >110 mg/dL is highly suggestive, whereas a level of <50 mg/dL excludes the diagnosis. The presence of pleural chylomicrons may further confirm the diagnosis of chylothorax (FIGURE 1).7,8,39,40
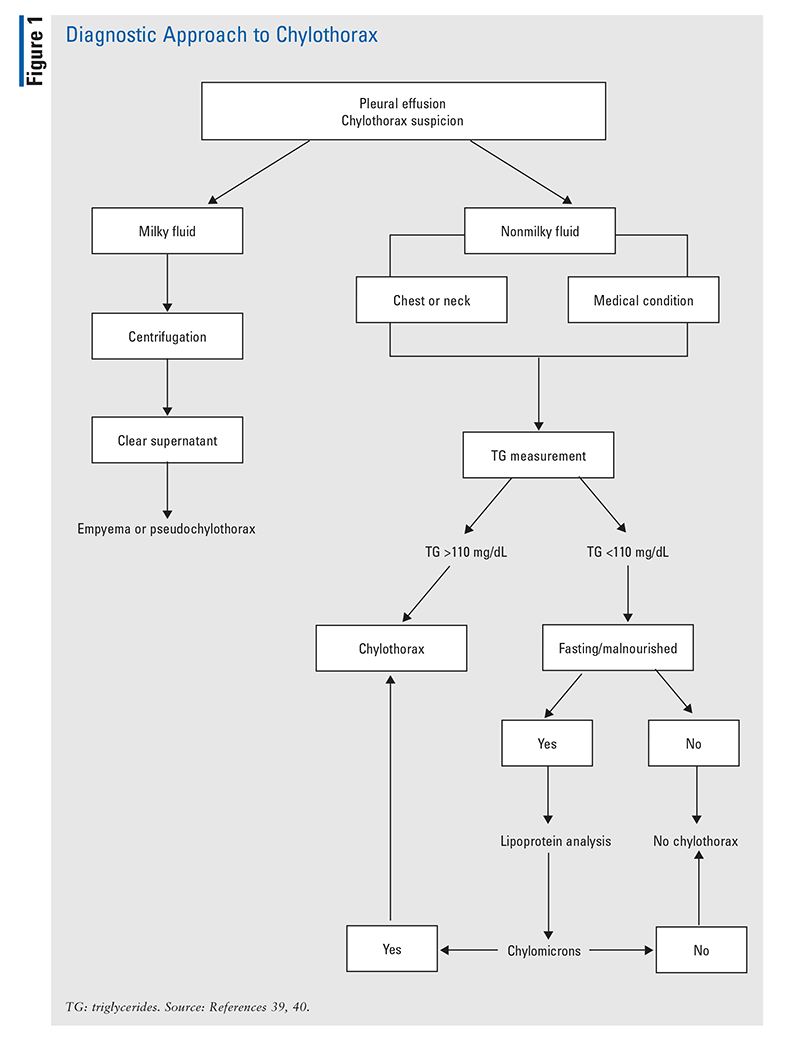
A lymphangiogram, which uses imaging to visualize the body’s lymphatic system, might be a helpful diagnostic tool in cases when chylothorax does not respond to conservative management and surgery is being considered.1,7,8,10,39,40 Lymphangiography involves injection of poppyseed oil into the lymphatic system (foot and ankle), with the flow of the contrast to the thoracic duct observed fluoroscopically. This process identifies the exact location of the lymphatic leaks. Although a success rate of 51% has been reported, complications such as pneumonia, oil embolization, wound infection, pulmonary edema, and urticaria have occurred. The complications are related to the amount of contrast injected (this should not exceed 14 mL).7
One of the most serious complications of chylothorax is malnutrition. Leakage of chyle and lymph into the pleural space can lead to the loss of essential proteins, vitamins, immunoglobulins, fat, electrolytes, and water. Patients with chylothorax present with respiratory-distress symptoms such as dyspnea, chest pain, cough, and fatigue, as in any pleural effusion. Immunosuppression may result from the leakage of immunoglobulins, lymphocytes, and proteins into the pleural space. Additionally, drugs such as digoxin, amiodarone, and cyclosporine are lost via the leaking chyle.6,5,39
TREATMENT
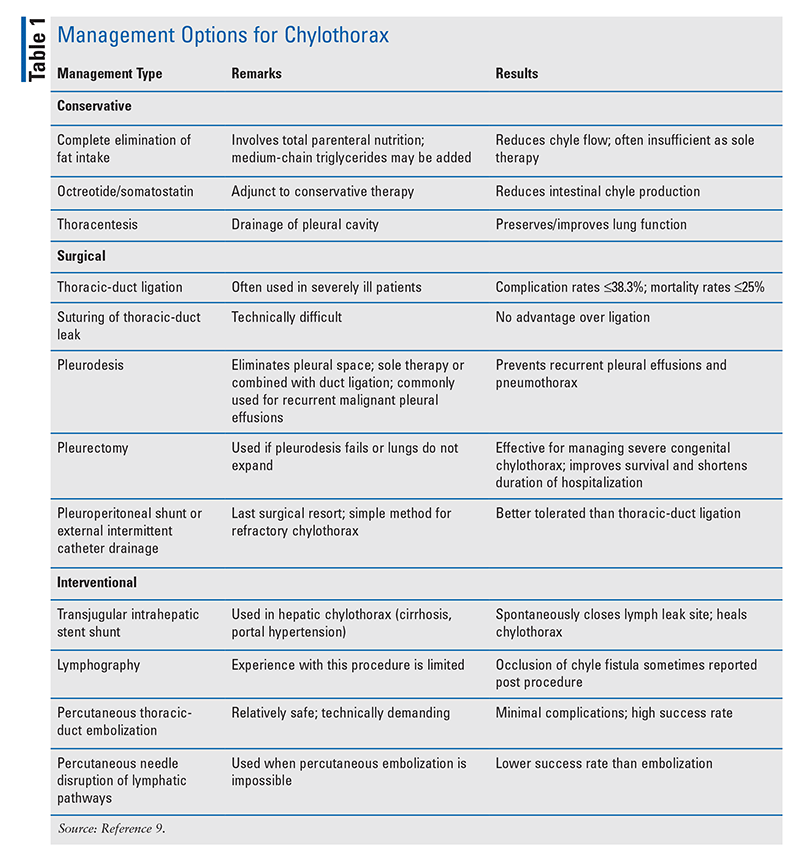
Given the rarity of chylothorax, prospective, randomized studies and evidence-based guidelines on how to treat it are lacking, and definitive management has not emerged in the literature. Which therapeutic approach to management—conservative, surgical, or interventional—is taken depends on the individual patient’s cause of chylothorax and accompanying clinical conditions (TABLE 1). Most researchers recommend conservative management in all patients initially, reserving invasive therapies for those in whom conservative management fails.
Conservative Management
Conservative management aims to reduce chyle flow, drain the pleural cavity, and prevent complications (malnutrition, sepsis, and immunosuppression). This approach is typically used first-line in patients with flow rates <500 mL/day and is associated with a favorable rate of spontaneous closure.9,40
Thoracentesis: Initial conservative management in symptomatic patients involves placement of a thoracostomy tube to drain the chyle from the pleural space, allow the lungs to expand, and relieve intrathoracic pressure. This may further improve the respiratory distress, reduce the pleural space, and seal the leak.9
Elimination of Fat Intake: To reduce chyle flow, total elimination of oral or enteral fat intake should be initiated. The effect of oral intake on the volume of the chyle leak is unpredictable and varies from patient to patient and from day to day. Therefore, in consultation with a dietitian, a nutritional strategy should be planned and modified daily. Clear liquids (e.g., water, fruit juice, nutritional beverages) are well tolerated, do not increase the volume of chyle leak, and should not be restricted. Uptake of orally administered micronutrients (fat-soluble vitamins, essential trace elements) may be suboptimal owing to the fat-free or low-fat diet. The oral low-fat diet is highly unpalatable and may lead to noncompliance. Oral intake may be gradually advanced to medium-chain triglycerides (MCTs), which are available commercially in liquid or capsule form. MCTs are preferable to longer-chain triglycerides because they pass directly into the portal vein, thus reducing chyle formation. Although MCTs are well tolerated, steatorrhea, mild gastrointestinal (GI) upset, and increased serum cholesterol have been reported with higher dosages as well as in cases of preexisting hyperlipidemia.11,38,40-43
Parenteral Nutrition: Finally, parenteral nutrition (PN) should be considered if all other options have failed and the patient is at risk for developing malnutrition. PN restores the nutritional deficits and balances metabolic impairments imposed by long-standing chylous leakage. Whereas many hospitals are equipped with ready-to-use PN containing all of the components, custom-made PN requires trained pharmacists and appropriate equipment, so the custom-made option may be impractical for some hospitals. Although it is simpler to immediately restrict or limit oral intake and place the patient on PN, this will be hard to justify given the risks and complications associated with PN (e.g., infections and GI atrophy) as well as the increased cost.44
Octreotide/Somatostatin: Evidence is increasing on the utility of octreotide, a synthetic long-acting analogue of somatostatin, as an adjunct to conservative management of chylothorax. Octreotide and somatostatin exert a broad range of inhibitory actions in many organs, including the liver, pancreas, and pituitary gland, as well as the central nervous system and GI tract. These agents inhibit secretion of several hormones or related peptides; they also suppress GI motility, gastric-acid production, pancreatic-enzyme secretion, and bile and colonic-fluid secretion. An important advantage of octreotide over somatostatin is that continuous IV infusion is not essential; although continuous IV infusion (6 mg/day) may be used, octreotide may also be administered via SC injection (50-100 mcg q8h). A long-acting form of octreotide is available for monthly IM administration. Evidence of the benefit in chylothorax management derives from numerous case reports and small retrospective studies. No prospective studies have confirmed or standardized the dosage, method, or duration of drug administration. In most patients, a significant reduction in chyle flow occurred during the first week of treatment; therefore, administration for 1 to 2 weeks is suggested. Octreotide is generally well tolerated, but mild and transient adverse effects include cramps, nausea, diarrhea, fatty stools, flatulence, hepatic and renal impairment, mild hyperglycemia (related to inhibited insulin secretion), and hypothyroidism (likely related to pituitary inhibition).40,42,45,46 Success rates with the use of conservative therapy range from 16% to 75% for low-output chylous leakage (<1,000 mL/day); however, with high-output leakage (>1,000 mL/day), the success rate is low.40,42,45,46
Surgical Management
Surgical treatment should be considered for patients in whom conservative management has failed, in order to avoid severe malnutrition. Surgery is indicated when >1,500 mL/day chyle is being drained or drain output reaches 1,000 mL/day for 5 days; a leak persists for >2 weeks; or the drain output remains unchanged over 1 to 2 weeks. Identifying the thoracic duct or the leak is the main difficulty with surgery. Surgical management is successful in about 90% of cases, although some patients (~11%) may undergo multiple procedures.7,47,48 TABLE 1 summarizes the available surgical options.
Pleurodesis: One of the accepted surgical options, especially in patients with malignant tumors, is pleurodesis. This procedure may be used as an alternative to or in conjunction with duct ligation, but it is effective only in patients with expandable lungs. Pleurodesis is performed to eliminate pleural space and prevent recurrent pleural effusions. The procedure can be chemical or mechanical. In chemical pleurodesis, a tube is inserted in the chest via a small incision to remove fluid from the pleural space. A substance (commonly medical talc, tetracycline, minocycline, bleomycin, or povidone iodine) is placed in the chest tube that causes the linings of the lung and chest wall to stick together. In mechanical pleurodesis, a thoracoscope is used to insert medical talc into the space around the lungs.9,40,43
Interventional Management
Interventional methods such as percutaneous thoracic duct ligation, thoracic duct embolization, or lymphatic-pathway disruption should be considered for patients in whom medication therapy has failed. All three procedures are safe, effective, and minimally invasive and can be used for both traumatic and nontraumatic chylothorax. The procedure’s success will depend on the etiology of the effusion, the volume of effusion, and the reduction rate of the effusion volume.49-52
DISCUSSION
Chylothorax is a rare cause of pleural effusion that most commonly results from obstruction or destruction of the thoracic duct. Often a patient will present with more than one diagnosis. The feature considered typical of chylothorax—milky appearance—is not always present. Diagnosis is based on clinical findings post surgery as well as radiologic confirmation of pleural effusion using chest x-ray, computed tomography, or ultrasound. Thoracentesis to analyze pleural fluid and chylomicrons will confirm the presence of chylothorax.1-5
Chylothorax results in the loss of fluid, electrolytes, proteins, fat, fat-soluble vitamins, and lymphocytes. Accordingly, chyle leakage may result in malnutrition, metabolic imbalances, and infections due to immunodeficiency. Furthermore, the accumulation of chyle in the pleural space may lead to lung compression and resultant cardiopulmonary compromise.3,4
The management of chylothorax is not clearly established, but conservative treatment is the most accepted therapeutic strategy. Management may be more successful in patients with low chyle output than in those with high output. An important conservative step is thoracic drainage to relieve respiratory symptoms, followed by nutritional modification to reduce chyle production. Octreotide, an alternative conservative option, may reduce chyle flow and promote closure of thoracic-duct leaks, and it is often used effectively in combination with dietary modifications to avoid surgical intervention. Surgical intervention may be necessary to repair the thoracic duct in the presence of persistent fistula, significant leakage, or associated complications. Surgical failure is a risk because of the technical and surgical manipulation of the fragile duct. It is generally agreed that surgery should be reserved for failure of conservative management. It is uncertain how long it takes to determine whether conservative treatment has been a failure, and it is not clear which patients are the best candidates for surgery. Whether surgical repair reduces hospital stay or potential complications is unknown. Finally, thoracic-duct embolization is an appealing alternative to surgical exploration, but it carries significant mortality and may not be an option in many centers.6-11
CONCLUSION
Chylothorax remains a rare complication of thoracic surgery or trauma, but the associated complications may result in increased morbidity and mortality. Clinicians encountering this condition should have a clear understanding of the pathophysiology of the chyle leak, as this will inform accurate diagnosis and the appropriate treatment course. Overall, management will depend on the clinical situation and the availability of local treatment options. Consultation with a dietitian and interventional radiology can contribute to a positive outcome. Overall, conservative management is quite successful.
REFERENCES
1. Teba L, Dedhia HV, Bowen R, Alexander JC. Chylothorax review. Crit Care Med. 1985;13:49-52.
2. Pérez J, Casal J, Rodríguez W. Always remember chylothorax. South Med J. 1999;92:833-835.
3. Merrigan BA, Winter DC, O’Sullivan GC. Chylothorax. Br J Surg. 1997;84:15-20.
4. Paes ML, Powell H. Chylothorax: an update. Br J Hosp Med. 1994;51:482-490.
5. Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg. 2007;32:362-369.
6. Valentine VG, Raffin TA. The management of chylothorax. Chest. 1992;102:586-591.
7. Riley LE, Ataya A. Clinical approach and review of causes of a chylothorax. Respir Med. 2019;157:7-13.
8. Pillay TG, Singh B. A review of traumatic chylothorax. Injury. 2016;47:545-550.
9. Schild HH, Strassburg CP, Welz A, Kalff J. Treatment options in patients with chylothorax. Dtsch Arztebl Int. 2013;110:819-826.
10. Golden P. Chylothorax in blunt trauma: a case report. Am J Crit Care. 1999;8:189-192.
11. Martucci N, Tracey M, Rocco G. Postoperative chylothorax. Thorac Surg Clin. 2015;25:523-528.
12. Mukherjee K, Chakrabarty U, Dasbakshi K, et al. Management of chylothorax after coronary artery bypass grafting: two case reports and review of literature. Indian J Chest Dis Allied Sci. 2016;58:131-134.
13. Zakhour BJ, Drucker MH, Franco AA. Chylothorax as a complication of aortocoronary bypass. Two case reports and a review of the literature. Scand J Thorac Cardiovasc Surg. 1988;22:93-95.
14. Smith JA, Goldstein J, Oyer PE. Chylothorax complicating coronary artery by-pass grafting. J Cardiovasc Surg (Torino). 1994;35:307-309.
15. Kanakis MA, Misthos P, Kokotsakis JN, Lioulias AG. Chylothorax complicating thoracic aortic surgery. J Cardiovasc Surg. 2011;26:410-414.
16. Twomey CR. Chylothorax in the adult heart transplant patient: a case report. Am J Crit Care. 1994;3:316-319.
17. Robinson K, Weinstein ES, Langsfeld M. Bilateral chylothorax following thoracic duct ligation: case report and review of literature. Ann Vasc Surg. 1996;10:390-395.
18. Misthos P, Kanakis MA, Lioulias AG. Chylothorax complicating thoracic surgery: conservative or early surgical management? Updates Surg. 2012;64:5-11.
19. Pêgo-Fernandes PM, Ebaid GX, Nouer GH, et al. Chylothorax after myocardial revascularization with the left internal thoracic artery. Arq Bras Cardiol. 1999;73:387-390.
20. Haneda R, Booka E, Ishii K, et al. Postoperative chylothorax with a duplicated left-sided thoracic duct: a case report and review of the literature. Gen Thorac Cardiovasc Surg. 2020;68:1350-1353.
21. Falode O, Hunt I, Young CP. Chylothorax after coronary artery bypass surgery. J R Soc Med. 2005;97:314-315.
22. Schactman M, Scott C, Glibbery-Fiesel DR, et al. Chylopericardium following aortic valve replacement and coronary artery bypass surgery: a case report and discussion. Am J Crit Care. 1994;3:313-315.
23. Zhang H, Dziegielewski PT, Romanovsky A, Seikaly H. Bilateral chylothorax following neck dissection: case report and systematic review of the literature. J Otolaryngol Head Neck Surg. 2012;41:e26-e30.
24. Tsukahara K, Kawabata K, Mitani H, et al. Three cases of bilateral chylothorax developing after neck dissection. Auris Nasus Larynx. 2007;34:573-576.
25. Srikumar S, Newton JR, Westin TAB. Bilateral chylothorax following left-sided radical neck dissection. J Laryngol Otol. 2006;120:705-707.
26. Lofrese G, Cultrera F, Visani J, et al. Chylothorax in spine fractures: a rarely reported complication? Literature review with an example case. J Trauma Acute Care Surg. 2020;89:e140-e146.
27. Moussa AM, Maybody M, Gonzalez-Aguirre AJ, et al. Thoracic duct embolization in post-neck dissection chylous leakage: a case series of six patients and review of the literature. Cardiovasc Intervent Radiol. 2020;43:931-937.
28. Ilczyszyn A, Ridha H, Durrani AJ. Management of chyle leak post neck dissection: a case report and literature review. J Plast Reconstr Aesthet Surg. 2011;64:e223-e230.
29. Prabhu V, Passant C. Left-sided neck dissection and chylothorax: a rare complication and its management. J Laryngol Otol. 2012;126:648-650.
30. Diaz-Guzman E, Culver DA, Stoller JK. Transudative chylothorax: report of two cases and review of the literature. Lung. 2005;183:169-175.
31. Zhang C, Zhang RM, Pan Y, et al. Late-onset chylothorax during chemotherapy after lobectomy for lung cancer: a case report and review of the literature. Medicine (Baltimore). 2019;98:e15909.
32. Rice TW, Milstone AP. Chylothorax as a result of chronic lymphocytic leukemia: case report and review of the literature. South Med J. 2004;97:291-294.
33. Merki V, Pichler J, Giger R, Mantokoudis G. Chylothorax in thyroid surgery: a very rare case and systematic review of the literature. J Otolaryngol Head Neck Surg. 2016;45:52.
34. Nagano N, Suzuki M, Tamura K, et al. Refractory chylothorax and lymphedema caused by advanced gastric cancer. Intern Med. 2019;58:3143-3148.
35. Gomes AO, Ribeiro S, Neves J, Mendonça T. Uncommon aetiologies of chylothorax: superior vena cava syndrome and thoracic aortic aneurysm. Clin Respir J. 2015;9:185-188.
36. Rajagopala S, Kancherla R, Ramanathan RP. Tuberculosis-associated chylothorax: case report and systematic review of the literature. Respiration. 2018;95:260-268.
37. Zhang L, Zu N, Lin B, Wang G. Chylothorax and hemopericardium in Behcet’s diseases: case report and literature review. Clin Rheumatol. 2013;32:1107-1111.
38. Underwood J, Buckley J, Manning B. Gorham disease: an intraoperative case study. AANA J. 2006;74:45-48.
39. McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010;104:1-8.
40. Skouras V, Kalomenidis I. Chylothorax: diagnostic approach. Curr Opin Pulm Med. 2010;16:387-393.
41. Bender B, Murthy V, Chamberlain RS. The changing management of chylothorax in the modern era. Eur J Cardiothorac Surg. 2016;49:18-24.
42. De Hert S, Heytens L, Van Hee R, Adriaensen H. Current management of traumatic chylothorax. Acta Anaesthesiol Belg. 1988;39:101-107.
43. Chalret du Rieu M, Baulieux J, Rode A, Mabrut JY. Management of postoperative chylothorax. J Visc Surg. 2011;148:e346-e352.
44. Sriram K, Meguid RA, Meguid MM. Nutritional support in adults with chyle leaks. Nutrition. 2016;32:281-286.
45. Ismail NA, Gordon J, Dunning J. The use of octreotide in the treatment of chylothorax following cardiothoracic surgery. Interact Cardiovasc Thorac Surg. 2015;20:848-854.
46. Kalomenidis I. Octreotide and chylothorax. Curr Opin Pulm Med. 2006;12:264-267.
47. Omloo JMT, Lagarde SM, Vrouenraets BC, et al. Compartimentalization for chylothorax originating from the abdomen after extended esophagectomy. Report of two cases and review of the literature. Dig Surg. 2006;23:86-92.
48. Kranzfelder M, Gertler R, Hapfelmeier A, et al. Chylothorax after esophagectomy for cancer: impact of the surgical approach and neoadjuvant treatment: systematic review and institutional analysis. Surg Endosc. 2013;27:3530-3538.
49. Lyon S, Mott N, Koukounaras J, et al. Role of interventional radiology in the management of chylothorax: a review of the current management of high output chylothorax. Cardiovasc Intervent Radiol. 2013;36:599-607.
50. Sieczka EM, Harvey JC. Early thoracic duct ligation for postoperative chylothorax. J Surg Oncol. 1996;61:56-60.
51. Crucitti P, Mangiameli G, Petitti T, et al. Does prophylactic ligation of the thoracic duct reduce chylothorax rates in patients undergoing oesophagectomy? A systematic review and meta-analysis. Eur J Cardiothorac Surg. 2016;50:1019-1024.
52. Marcon F, Irani K, Aquino T, et al. Percutaneous treatment of thoracic duct injury. Surg Endosc. 2011;25:2844-2848.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





