ABSTRACT: Atrial fibrillation (AF) is a supraventricular tachyarrhythmia that can cause stroke/systemic embolism (SE), and therefore, anticoagulation may be indicated. Patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) may have greater risk for AF, stroke/SE, and bleeding, so the net benefit of anticoagulation is controversial. Evaluation of published data on oral anticoagulation in patients with CKD/ESRD shows that overall, warfarin has been associated with an increased risk for stroke and death, compared with no anticoagulation. Compared with warfarin users, apixaban users were shown to have significantly lower risk for stroke/SE, death, and major bleeding, but no difference was seen for efficacy outcomes when compared with no anticoagulation. The overall benefit-risk ratio must be carefully analyzed in this high-risk patient population, and the decision to anticoagulate must be conducted in a patient-specific manner.
US Pharm. 2021;45(3)36-40.
Atrial fibrillation (AF) is the most common sustained arrhythmia. It is a supraventricular tachyarrhythmia, involving uncoordinated atrial activation and, subsequently, ineffective atrial contraction. Given this, left atrial blood stasis may occur, particularly in the left atrial appendage, thereby leading to stroke/systemic embolism (SE).1,2 To decrease the risk for stroke/SE, anticoagulation is administered with warfarin and direct-acting oral anticoagulants (DOACs), such as apixaban, edoxaban, dabigatran, and rivaroxaban. Unlike warfarin, DOACs are limited for use in patients with nonvalvular AF only.
According to the American College of Cardiology (ACC) and American Heart Association (AHA), risk stratification with scoring criteria such as the CHA2DS2-VASc and HAS-BLED may be used to determine the risk for stroke and bleeding, respectively, when determining the risks versus benefits of anticoagulation for patients with AF.3 However, it is important that the identified risk factors for bleeding in HAS-BLED were primarily among warfarin users, so the criteria is not entirely applicable in DOAC users. Additionally, patients with end-stage renal disease (ESRD) undergoing dialysis were excluded in the generation and validation of these risk scores. They were also excluded from randomized controlled trials for DOACs. It is pivotal to note that patients with chronic kidney disease (CKD) and ESRD have significantly greater risk for AF, stroke/SE, and bleeding, given the pathophysiology and nature of the disease.2,4 Possible explanations for these risks include frequent heparin exposure, repeated vascular access, and abnormal blood coagulation, as well as calcification.
Traditionally, the drug of choice for CKD/ESRD patients has been warfarin. The reasoning for such therapy is that warfarin undergoes extensive hepatic metabolism into inactive compounds, theoretically posing less risk to the renally impaired patient. Aside from warfarin, apixaban is now approved for use in ESRD for stroke prevention in AF and treatment of venous thromboembolism. Additionally, the lower limit for creatinine clearance has been adjusted for rivaroxaban for stroke prevention in AF, thereby permitting use in the ESRD population. The remaining two DOACs, dabigatran and edoxaban, are approved for use with dosage adjustment in moderate-severe CKD for stroke prevention.5 However, the net benefit of anticoagulation with apixaban or warfarin in the ESRD population is controversial.
A database search was conducted by the author on PubMed (1946–2020), Embase (1947–2020), and Wiley Cochrane Library—Central Register of Controlled Trials (1996–2020) to search for clinical trials on oral anticoagulants in CKD/ESRD. Search strategies included controlled vocabulary and terms such as “oral anticoagulation,” “apixaban,” “dabigatran,” “edoxaban,” and “rivaroxaban.” No limits on year were placed, and only studies published in English were included. ClinicalTrials.gov and the International Clinical Trials Registry Platform were searched to find pertinent clinical trials. Abstracts and conference papers from relevant society meetings such as the ACC and AHA were included. Efficacy and safety outcomes for warfarin and DOACs in the CKD/ESRD population were assessed and included. Articles excluded were reviews or editorials, case reports, and studies involving pharmacokinetics, patients without CKD/ESRD, or patients with a history of kidney transplantation.
REVIEW OF STUDIES ON ORAL ANTICOAGULATION IN CKD/ESRD AND AF
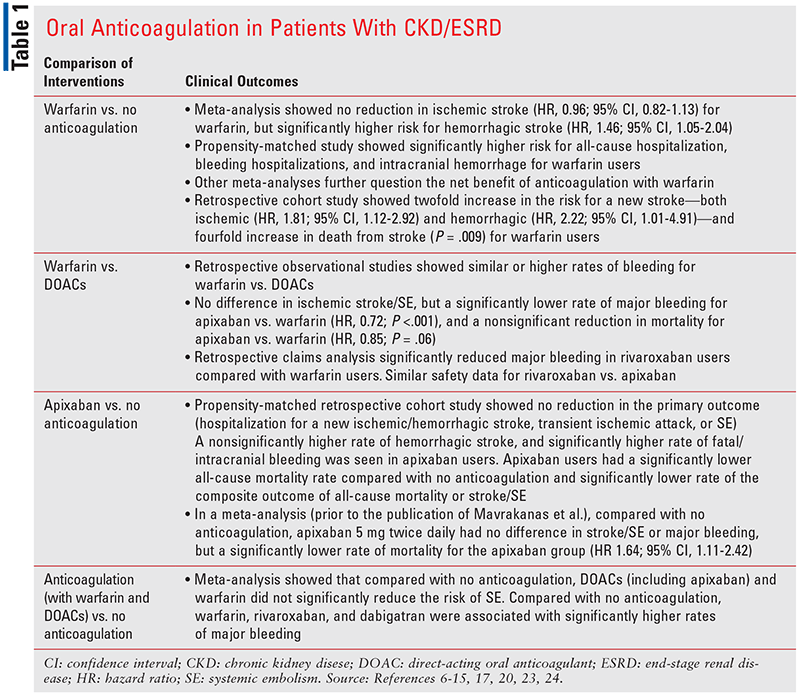
A summary of available clinical outcomes for oral anticoagulation in patients with ESRD and AF is presented in Table 1.
Warfarin
In a meta-analysis of primarily retrospective observational studies, patients with ESRD and AF were compared with and without warfarin.6 Warfarin was not associated with a reduction in ischemic stroke (hazard ratio [HR], 0.96; 95% confidence interval [CI], 0.82-1.13) but led to a significantly higher risk for hemorrhagic stroke (HR, 1.46; 95% CI, 1.05-2.04). However, there was significant heterogeneity among the studies. The time in therapeutic range (TTR), which is an acceptable indicator to assess the level of efficacy for warfarin, was not reported.
Furthermore, a recent propensity-matched study of 8,410 ESRD patients with AF using a Medicare-claims database found a significantly higher risk for all-cause hospitalization, bleeding hospitalizations, and intracranial hemorrhage for warfarin users versus nonwarfarin users.7 Other meta-analyses further question the net benefit of anticoagulation with warfarin, though their inherent limitations must be considered, such as the nonsimilar risks for bleeding and stroke/SE across the studies.8,9
Moreover, when a retrospective cohort study of 1,671 ESRD patients with AF compared outcomes in warfarin users (44.7%) versus nonusers, warfarin users had a twofold increase in the risk for a new stroke—both ischemic (HR, 1.81; 95% CI, 1.12-2.92) and hemorrhagic (HR, 2.22; 95% CI, 1.01-4.91)—and a fourfold increase in death from stroke (P = .009) in a mean follow-up of 1.6 years.10 Variables remained significant with covariate and propensity-score adjustment models. The increase in stroke risk was associated with increasing international normalized ratios (INRs), with the highest risk for stroke seen in patients without INR monitoring in the first 90 days of dialysis. The TTR was not reported in this study, and outcome data for patients on concomitant antiplatelets were not reported.
The possibly increased rate of stroke and death for warfarin may be explained by warfarin-induced calciphylaxis.11 Matrix G1a is an endogenous, vitamin K–dependent protein that directly inhibits calcium deposition in the arteries. Upon inhibition, calciphylaxis of the arterial vessel wall may develop, causing ischemic events and mortality. This occurrence has been reported even in patients with normal renal function, and ESRD patients have vascular calcification at baseline, primarily due to hyperphosphatemia.
Warfarin Versus Apixaban
Building on earlier studies showing an increased rate of bleeding in ESRD patients taking warfarin versus no anticoagulation, more recent studies have compared warfarin with DOACs. Warfarin was associated with a higher rate of bleeding when compared with DOACs, while some studies showed comparable safety data for warfarin with apixaban.11-15 It has been reported that ESRD patients who develop calciphylaxis on warfarin may be safely and effectively switched to apixaban as an alternative.16
In a retrospective cohort study of Medicare beneficiaries with ESRD on hemodialysis (HD) (n = 25,523) by Siontis and colleagues, 91% were taking warfarin, and of the apixaban users, 44% were taking 5 mg twice daily.17 The apixaban dose was unable to be assessed for appropriateness due to lack of data on age and body weight. The main analysis was performed in prognosis-matched cohorts after an average time of 105 and 157 days on apixaban and warfarin, respectively. Patients were followed up until death or drug discontinuation. When the apixaban and warfarin groups were compared, there was no difference in ischemic stroke/SE, but there was a significantly lower rate of major bleeding for patients taking apixaban (HR, 0.72; P <.001), as well as a nonsignificant reduction in mortality for apixaban (HR, 0.85; P = .06).17
Furthermore, in sensitivity analyses, compared with the apixaban 2.5-mg twice-daily group, the apixaban 5-mg twice-daily group had a significantly lower risk for ischemic stroke/SE (HR, 0.61; P = .04) and lower death rate (HR, 0.64; P = .01).17 Given the lack of data on age and body weight, it may be possible that in-group differences persisted between the apixaban 5- mg and 2.5-mg twice-daily users. Perhaps healthier patients with a lower risk of bleeding were given the 5- mg dose of apixaban. Moreover, compared with the warfarin group, the apixaban 5-mg twice-daily group had a significantly lower risk for ischemic stroke/SE (HR, 0.64; P = .04), death rate (HR, 0.63; P = .003), and major bleeding (HR, 0.71; P = .02). However, the follow-up periods were relatively short, suggesting lack of long-term tolerability in this patient group. Also, the annual intracranial hemorrhage (ICH) rate of 3.1% was much higher compared with historical rates, such as 0.33% in the ARISTOTLE trial.17
In contrast, despite the reduction in bleeding and mortality seen by Siontis and colleagues, patients with ESRD taking apixaban were found to have unacceptably high rates of bleeds, especially those with risk factors such as higher number of HD sessions, greater cumulative exposure to apixaban, and higher daily dose of apixaban.18,19 Most importantly, Mavrakanas and colleagues found results that shed pivotal insights on apixaban versus no anticoagulation. In a retrospective cohort study using the 2012–2015 U.S. Renal Data System, propensity-score matching was performed to compare AF patients on dialysis treated with apixaban (n = 521) or no anticoagulation (n = 1,561).20 Patients were matched for baseline characteristics including CHA2DS2-VASc and a modified HAS-BLED score. The primary outcome was hospitalization for a new ischemic/hemorrhagic stroke, transient ischemic attack, or SE. Patients were followed up until time of death, kidney transplantation, or study termination. Apixaban did not have an association with a reduction in the primary outcome compared wth no anticoagulation (7.5 vs. 7 events per 100 patient-years; HR, 1.2; P = .47). However, a nonsignificantly higher rate of hemorrhagic stroke was seen in apixaban users versus no anticoagulation.
Apixaban users had a significantly lower all-cause mortality rate compared with no anticoagulation (HR, 0.58; 95% CI, 0.43-0.78) and a significantly lower rate of the composite outcome of all-cause mortality or stroke/SE (HR, 0.56; 95% CI, 0.41-0.76).20 However, apixaban users had a significantly higher rate of fatal/intracranial bleeding compared with no anticoagulation (HR, 5.1; P <.001), and this rate was significantly higher when comparing the apixaban dose of 5 mg twice daily compared with no anticoagulation (HR, 4.61; 95% CI, 1.91-11.15). Compared with no anticoagulation, apixaban users who were given 5 mg twice daily also had a significantly higher rate of the primary outcome and hemorrhagic stroke. Given that apixaban users had significantly higher rates of bleeding without a reduction in stroke/SE, the net benefit of anticoagulation in dialysis patients is questionable.
Furthermore, the rate of clinically important bleeding was not significantly different between apixaban users and those without anticoagulation (59.2 vs. 56.9 per 100 patient-years; HR, 1.15; P = .26), showing that patients in this population likely have a higher baseline risk of bleeding even without anticoagulation.20 The lower mortality rates reported in this study, despite propensity-score matching, may be attributed to patients on anticoagulation being potentially healthier at baseline compared with patients for whom anticoagulation was deferred; all differences in variables may not have been accounted for, and residual confounding may have persisted. Lastly, there was lack of data on body weight, and thus, appropriateness of the apixaban dose was unable to be assessed.
Other DOACs
Regarding other DOACs, edoxaban has not been studied in the ESRD/HD population, and dabigatran is not approved for use due to an increased risk of hemorrhagic death, major bleeding, and stroke compared with warfarin.21 These poor outcomes may be related to the fact that 85% of dabigatran is renally excreted as active metabolites, and up to 60% of the drug is dialyzable. Thus, HD patients may have an exceedingly high accumulation of the drug pre-HD, and significantly low concentrations post-HD. For rivaroxaban, a retrospective claims analysis of stage IV-V CKD/HD patients with AF showed significantly reduced major bleeding in rivaroxaban users compared with warfarin users (HR, 0.68; 95% CI, 0.47-0.99) at a mean follow-up of 1.4 (0.6-2.7) years, though TTR was not assessed.22 Also, there was a nonsignificant reduction in stroke/SE. Thus, rivaroxaban 15 mg daily is approved for patients with a creatinine clearance of less than 15 mL/min, which includes the ESRD population. Safety for rivaroxaban has shown to be similar to apixaban in ESRD patients at a follow-up of 0.87 (0.38, 1.56) years.23
With increasing data on DOACs in ESRD and AF, a meta-analysis of 16 nonrandomized, observational studies was conducted by Kuno and colleagues to further examine the net clinical benefit of anticoagulation.24 All but two studies compared warfarin versus no anticoagulation; one involved rivaroxaban and dabigatran versus warfarin, and one involved apixaban versus warfarin. Overall, DOACs (including apixaban) and warfarin did not significantly reduce the risk of SE in patients with ESRD and AF versus no anticoagulation. Compared with no anticoagulation, warfarin was associated with a significantly higher rate of major bleeding. Compared with no anticoagulation, rivaroxaban and dabigatran were associated with significantly higher rates of major bleeding. In the pooled analysis of no anticoagulation versus apixaban 5 mg twice daily, there was no difference in stroke/SE or major bleeding, but there was a significantly lower rate of mortality for the apixaban group (HR, 1.64; 95% CI, 1.11-2.42). Of note, this meta-analysis did not include the study by Mavrakanas and colleagues.
Further well-designed, randomized, controlled trials are needed to delineate the role of anticoagulation in the CKD/ESRD population. The results of the RENAL-AF trial were recently released.25 This study randomized patients with ESRD on HD and AF to apixaban (both 5 mg and 2.5 mg twice daily) (n = 82) and warfarin (n = 72). The trial was stopped early due to slow accrual of participants and possible loss of power. TTR for warfarin was 44.3%, and at the time, there was no difference between apixaban and warfarin in the primary outcome of clinically relevant nonmajor bleed (31.5% vs. 25.5%, P >.05). There are ongoing clinical trials that are anticipated to provide further clarification on anticoagulation in the CKD/ESRD population. AXADIA is an open-label, randomized, controlled trial to compare apixaban 2.5 mg twice daily with warfarin in patients with AF and ESRD on HD, and follow-up is planned for 24 months.26 SAFE-D is an open-label randomized trial of patients with ESRD on dialysis and AF to compare three arms: apixaban (both 5 mg and 2.5 mg twice daily), warfarin, and no anticoagulation, for 26 weeks.27 TTR will be assessed in both trials. It is anticipated that these upcoming trials would further characterize the role of oral anticoagulation in CKD/ESRD patients and help ascertain the most appropriate drug therapy in this population.
DISCUSSION AND CONCLUSIONS
Although the CHA2DS2-VASc and HAS-BLED scoring criteria are used in the general population to weigh the risks against the benefits of anticoagulation, these scoring criteria are not valid in the CKD/ESRD population. Furthermore, CKD/ESRD patients may have inherent risks for bleeding and stroke/SE, and as such, the net benefit of anticoagulation in patients with CKD/ESRD and AF is unclear. An individualized risk-benefit assessment must be made for each patient. Based on available data, if the decision is to anticoagulate, warfarin should likely be avoided due to an increased risk for bleeding, stroke, and death. Though apixaban has shown a reduction in bleeding and mortality in studies compared to warfarin, the study by Mavrakanas and colleagues brings to light the possibly higher net benefit of forgoing anticoagulation, compared to anticoagulation with apixaban in the ESRD population.
The main limitation of this review is that the studies included were nonrandomized and observational in nature, and as such, selection bias, confounders, and questionability in record-keeping may limit the generalizability of these studies. Associations can be made based on the studies, but causation cannot be assumed. There was also significant heterogeneity in the studies. Until larger, well-designed, randomized, controlled studies are conducted, the net benefit of anticoagulation in the CKD/ESRD population remains unknown.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895-900.
2. Soliman EZ, Prineas RJ, Go AS, et al; Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 2010;159:1102-1107.
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society [published correction appears in Circulation. 2014 2;130(23):e272-4]. Circulation. 2014;130:e199:e267.
4. Winkelmayer WC, Patrick AR, Liu J, et al. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22:349-357.
5. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125-e151.
6. Randhawa MS, Vishwanath R, Rai MP, et al. Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e202175.
7. Pokorney SD, Black-Maier E, Hellkamp AS, et al. Oral anticoagulation and cardiovascular outcomes in patients with atrial fibrillation and end-stage renal disease. J Am Coll Cardiol. 2020;75:1299-1308.
8. Liu G, Long M, Hu X, et al. Effectiveness and safety of warfarin in dialysis patients with atrial fibrillation: a meta-analysis of observational studies. Medicine (Baltimore). 2015;94:e2233.
9. Nochaiwong S, Ruengorn C, Awiphan R, et al. Efficacy and safety of warfarin in dialysis patients with atrial fibrillation: a systematic review and meta-analysis. Open Heart. 2016;3:e000441.
10. Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223-2233.
11. Saifan C, Saad M, El-Charabaty E, El-Sayegh S. Warfarin-induced calciphylaxis: a case report and review of literature. Int J Gen Med. 2013;6:665-669.
12. Wang X, Tirucherai G, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56:628-636.
13. Herndon K, Guidry TJ, Wassell K, Elliott W. Characterizing the safety profile of apixaban versus warfarin in moderate to severe chronic kidney disease at a Veterans Affairs hospital. Ann Pharmacother. 2020;54(6):554-560.
14. Schafer JH, Casey AL, Dupre KA, Staubes BA. Safety and efficacy of apixaban versus warfarin in patients with advanced chronic kidney disease. Ann Pharmacother. 2018;52(11):1078-1084.
15. Davis E, Darais D, Fuji K, et al. Prescribing and safety of direct-acting oral anticoagulants compared to warfarin in patients with atrial fibrillation on chronic hemodialysis. Pharmacy (Basel). 2020;8(1):37.
16. Garza-Mayers AC, Shah R, Sykes DB, et al. The successful use of apixaban in dialysis patients with calciphylaxis who require anticoagulation: a retrospective analysis. Am J Nephrol. 2018;48(3):168-171.
17. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138:1519-1529.
18. Bowie M, Valencia V, Perez-Alvarez I, Tran MH. Safety analysis of apixaban versus warfarin in patients with advanced kidney disease. J Thromb Thrombolysis. 2018;46(2):246-252.
19. Steuber TD, Shiltz DL, Cairns AC, et al. A multicenter analysis of factors associated with apixaban-related bleeding in hospitalized patients with end-stage renal disease on hemodialysis. Ann Pharmacother. 2017;51(11):954-960.
20. Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrology. 2020;15(8):1146-1154.
21. Chan KE, Edelman ER, Wenger JB, et al. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131:972-979.
22. Coleman CI, Kreutz R, Sood NA, et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. 2019;132:1078-1083.
23. Miao B, Sood N, Bunz TJ, Coleman CI. Rivaroxaban versus apixaban in non-valvular atrial fibrillation patients with end-stage renal disease or receiving dialysis. Eur J Haematol. 2020;104(4):328-335.
24. Kuno T, Takagi H, Ando T, et al. Oral anticoagulation for patients with atrial fibrillation on long-term hemodialysis. J Am Coll Cardiol. 2020;75:273-285.
25. Pokorney SD. RENal hemodialysis patients ALlocated apixaban versus warfarin in Atrial Fibrillation - RENAL-AF. Presented at the American Heart Association Annual Scientific Sessions (AHA 2019), Philadelphia, PA, November 16, 2019.
26. Compare apixaban and vitamin-k antagonists in patients with atrial fibrillation (AF) and end-stage kidney disease (ESKD) (AXADIA). ClinicalTrials.gov. October 14, 2016. https://clinicaltrials.gov/ct2/show/NCT02933697. Accessed October 12, 2020.
27. Strategies for the management of atrial fibrillation in patients receiving dialysis (SAFE-D). ClinicalTrials.gov. June 17, 2019. https://clinicaltrials.gov/ct2/show/NCT03987711. Accessed October 12, 2020.
To comment on this article, contact rdavidson@uspharmacist.com.