US Pharm. 2021;46(12):HS10-HS16.
ABSTRACT: Guidelines for the management of adults with Clostridioides difficile infection (CDI) were recently published by the American College of Gastroenterology, followed by a focused update by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America (IDSA-SHEA). Recommended treatment is based on the type of episode (primary or recurrent) as well as the number of previous recurrences and/or presence of fulminant infection. The IDSA-SHEA guidelines suggest fidaxomicin as the preferred agent for treating initial CDI, with oral vancomycin considered an acceptable alternative. For a first recurrence, standard or extended-pulsed dosing of fidaxomicin is suggested, with oral vancomycin considered an acceptable alternative. Pharmacists are in a key position to recommend appropriate antimicrobial therapy and preventive measures for CDI management.
Clostridioides difficile (formerly known as Clostridium difficile) is an anaerobic, gram-positive, spore-forming rod that causes up to 25% of cases of antibiotic-associated diarrhea.1-3 The CDC has classified C difficile as an urgent threat, defined as a public-health threat that requires aggressive action.3 Although there has been a decline in healthcare-associated C difficile infection (CDI), an estimated 223,900 cases in hospitalized patients and 226,400 cases of community-associated CDI were reported in the United States in 2017.3,4 In 2021, clinical practice guidelines for CDI were updated by the American College of Gastroenterology (ACG), and the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America (IDSA-SHEA) published clinical practice guidelines focusing on the use of fidaxomicin and bezlotoxumab in adults.5,6 This article includes information from these guidelines, with an emphasis on the IDSA-SHEA treatment recommendations.
RISK FACTORS
A number of risk factors for CDI have been identified (TABLE 1).5,7-12 The main risk factors for development of CDI are exposure to the healthcare environment, advanced age (65 years or older), and exposure to antibiotics.5,7 Receipt of an antimicrobial agent is the most significant modifiable risk factor for initial or recurrent CDI.13 Although most antibiotics can disrupt normal intestinal gut flora, thereby creating an environment that enables growth and colonization of C difficile, carbapenems, clindamycin, fluoroquinolones, piperacillin-tazobactam, and third- and fourth-generation cephalosporins have been shown to confer the highest risk of infection.8,13 Patients are at highest risk for CDI during antimicrobial therapy and within the first month after its discontinuation, and they continue to be at risk for 3 months after completion of therapy.14
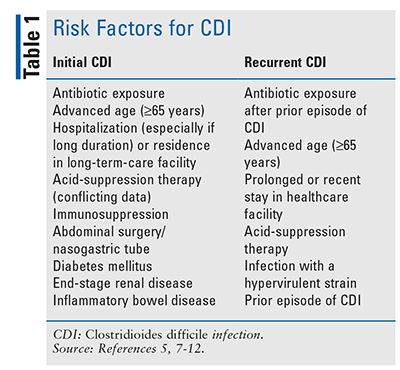
It is unclear whether the use of acid-suppressing medications such as proton pump inhibitors (PPIs) increase a patient’s risk for CDI, as some studies have shown an epidemiologic association but others have not; additional studies are needed to confirm causality.8,9,11-13 The guidelines state that unnecessary PPIs should always be discontinued but that there is not enough evidence to discontinue a PPI for prevention of CDI.5,13
Recurrent CDI may occur after completion of treatment, and approximately 25% of patients with a first episode of CDI will have a recurrent infection.10,13,15,16 The risk of recurrence increases with the number of CDI episodes, with up to 45% of patients experiencing recurrent CDI after the second episode and more than 60% having a recurrence after three or more episodes.10,16
Clinical Presentation and Classification
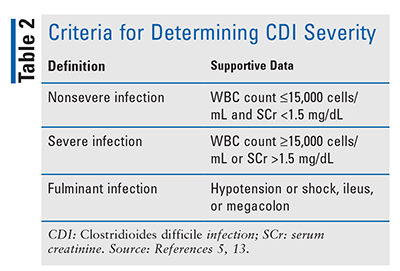
Clinical manifestations of CDI vary from asymptomatic carriage of the organism to mild or moderately acute watery diarrhea to severe colitis; potential life-threatening complications include sepsis, renal failure, toxic megacolon, and bowel perforation.7,8 Criteria for the definitions of nonsevere, severe, and fulminant CDI are presented in TABLE 2.5,13
DIAGNOSIS
Because colonization with C difficile is common, it is recommended that testing be performed only in patients with symptoms consistent with active CDI, which is characterized by unexplained new-onset diarrhea (i.e., three or more unformed stools within 24 hours).5,13 A two-step testing algorithm should be used to increase the accuracy of diagnosis of active CDI.5 The ACG guidelines recommend starting with a sensitive test, such as nucleic acid amplification testing (NAAT) or glutamate dehydrogenase (GDH). If the initial test is negative, then the patient does not have active CDI. If the initial test is positive, additional testing is recommended owing to limitations in the use of NAAT or GDH. Although NAAT is sensitive for detecting the presence of toxigenic strains of C difficile, it cannot distinguish between colonization and active production of toxin. The absence of GDH is strongly predictive of the absence of CDI; however, this enzyme may be produced by other clostridial species as well as by non–toxin-producing strains of C difficile. Therefore, if the result of one of these initial tests is positive, then a highly specific test, such as an enzyme immunoassay (EIA) that detects C difficile toxins A and B, should be used. If that result is also positive, the patient is diagnosed with active CDI. Discordant results (i.e., the initial sensitive test is positive and the EIA is negative) require further clinical evaluation to determine whether the patient has active CDI.5
TREATMENT
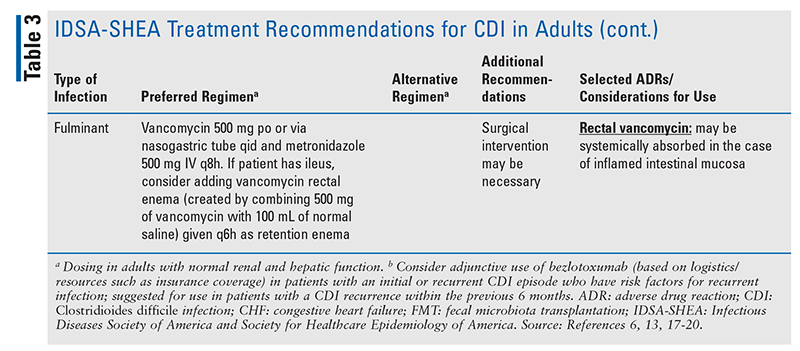
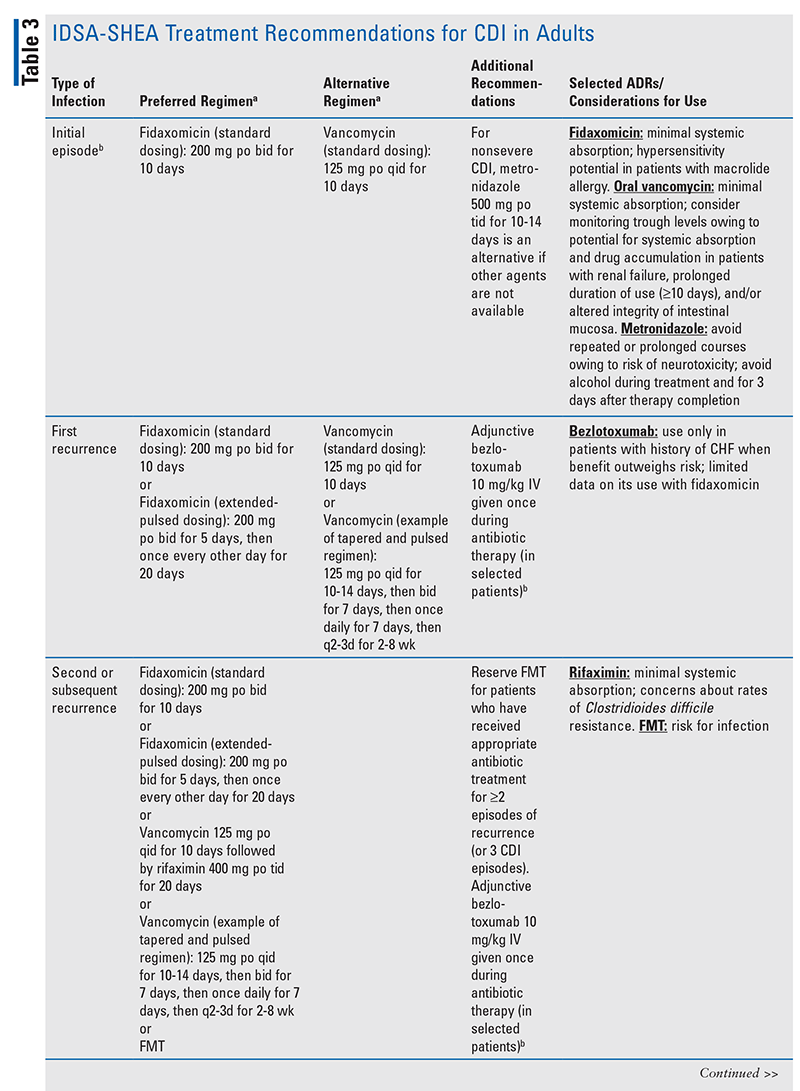
The IDSA-SHEA recommendations for CDI treatment and dosing regimens, selected adverse effects, and considerations for the use of CDI agents are presented in TABLE 3.6,13,17-20 The antimicrobial agent(s) that may have led to CDI should be discontinued, if possible, in order to decrease the risk of CDI recurrence; additionally, any fluid or electrolyte imbalances occurring as a result of CDI should be corrected.9,13,21
First Episode
The IDSA-SHEA guidelines state that fidaxomicin for 10 days at a standard dosing regimen is the preferred treatment for an initial episode of CDI; this recommendation is conditional, with a moderate certainty of evidence. Oral vancomycin for 10 days at a standard dosing regimen is an acceptable alternative.6
Recurrent CDI
First Recurrence: The IDSA-SHEA guidelines suggest using fidaxomicin as a standard or extended-pulsed dosing regimen over vancomycin in patients with a first recurrence of CDI; this is a conditional recommendation with a low certainty of evidence. The guidelines also state that vancomycin (either standard dosing or a tapered and pulsed regimen) is an acceptable alternative.6
Multiple Recurrences: The IDSA-SHEA guidelines include several treatment options for patients with multiple (i.e., two or more) recurrences of CDI. Additionally, fecal microbiota transplantation (FMT) is an option for those with multiple recurrences; however, it is recommended that FMT be reserved for patients who have received appropriate antibiotic treatment for at least two episodes of recurrence (or three CDI episodes). This is because of the potential for adverse events such as transmission of pathogenic organisms, including Escherichia coli and severe acute respiratory syndrome coronavirus 2.6
Fulminant CDI
Recommended treatment of fulminant CDI (characterized by hypotension or shock, ileus, or megacolon) has not changed from the previous version of the guidelines.6,13 Importantly, there is no current evidence to support the use of fidaxomicin for fulminant infections, so this agent is not recommended for fulminant CDI.6 Patients with fulminant CDI may also require surgical management.6,8
ADJUNCTIVE AGENTS
Bezlotoxumab
Bezlotoxumab is a humanized monoclonal antibody that binds to and neutralizes C difficile toxin B.6,20 It was approved in 2016 as a single-dose infusion in conjunction with antibiotic therapy for CDI to reduce recurrent CDI in adults at high risk for recurrence.6,20 The IDSA-SHEA guidelines suggest the addition of bezlotoxumab to antibiotic therapy (given at any time during antibiotic therapy) in patients who had a CDI recurrence within the previous 6 months.6 Patients with a first episode of CDI who have additional risk factors (particularly those with multiple risk factors) for recurrence, such as age 65 years or older, immunocompromised status, or severe CDI, may benefit from the addition of bezlotoxumab to antibiotic therapy in settings with available resources for its use.6,15
It is important to note that bezlotoxumab carries a warning of heart failure based on higher rates of heart failure reported in patients taking bezlotoxumab compared with placebo; this adverse event occurred primarily in patients with a history of congestive heart failure (CHF).20 Therefore, in patients with a history of CHF, bezlotoxumab should be used only when the benefit outweighs the risk.6,20 The IDSA-SHEA guidelines acknowledge that most studies of bezlotoxumab with antibiotics used vancomycin or metronidazole and that data on its combination with fidaxomicin are limited.6 Additionally, the use of this agent may be limited by logistical factors such as insurance coverage, particularly in patients with a first episode of CDI.6
Probiotics
According to the 2020 American Gastroenterological Association (AGA) guidelines on the role of probiotics in managing gastrointestinal (GI) disorders, probiotics should be used only in the context of a clinical trial on the treatment of patients with CDI. This is based on a knowledge gap, and standardized studies are needed in order to more clearly identify which specific probiotics are beneficial and which populations are most appropriate for their use.22
MONITORING
Patients should be monitored for resolution of signs and symptoms of infection, and they should be counseled regarding the risk of infection recurrence.13,16 Repeat testing (within a 7-day period) of stool during the same episode of diarrhea or after treatment to confirm infection eradication is not recommended.13 However, recurrent infection should be considered (with appropriate testing performed) in patients who develop new or worsening diarrhea after completing successful treatment for CDI.13,16 Selected side effects and usage considerations for agents used to treat CDI are listed in TABLE 3.6,13,17-20
PREVENTION
A multifactorial approach is recommended for the prevention of CDI. This includes infection prevention measures (e.g., hand hygiene, isolation precautions, contact precautions, and appropriate environmental cleaning and disinfection) as well as implementation of antimicrobial stewardship programs that restrict high-risk antibiotics and focus on minimizing the use and duration of unnecessary antimicrobial agents.5,13,21
Vancomycin for Suppression or Prophylaxis
The updated ACG guidelines state that the use of oral vancomycin as prophylaxis (to prevent recurrence) may be considered in patients with a recent history of CDI who require antibiotic treatment and are at high risk for recurrent infection (i.e., aged 65 years or older or significantly immunocompromised and hospitalized within the previous 3 months for severe CDI); this is a conditional recommendation with a low quality of evidence. The suggested dosage of vancomycin for prophylaxis is 125 mg orally once daily, continued for 5 days after completion of antibiotic therapy.5
Additionally, long-term suppression with oral vancomycin may be used in patients with recurrent CDI who are not candidates for FMT, developed a recurrence after FMT, or require antibiotics (either ongoing use or frequent courses); this recommendation is conditional with a very low quality of evidence. The suggested dosage of vancomycin for chronic suppression is 125 mg orally once daily.5
Recommendations on Probiotics
The ACG guidelines advise against the use of probiotics for primary prevention in patients receiving antibiotics or for secondary prevention of CDI recurrence.5 However, regarding their role in managing GI disorders, the AGA guidelines suggest that probiotics may be used in patients receiving antibiotics in order to prevent CDI; this is a conditional recommendation with a low quality of evidence.22 The AGA guidelines recommend using specific strains and combinations of strains (over no probiotics or other probiotic agents), including Saccharomyces boulardii; a combination of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R; a 3-strain combination of L acidophilus, Lactobacillus delbrueckii subspecies bulgaricus, and Bifidobacterium bifidum; or a combination of the previously listed 3-strain probiotics plus a fourth agent, Streptococcus salivarius subspecies thermophilus. Acknowledging that the beneficial effects of probiotics were demonstrated mainly in patients at very high risk for developing CDI, the AGA guidelines state that it is reasonable to not use probiotics for CDI prevention in patients (especially those in the outpatient setting) who have a low risk of CDI or those (particularly if they are immunocompromised) who want to avoid either the cost or the potential harms of probiotics.22
THE PHARMACIST’S ROLE
Pharmacists are in a key position to educate patients and healthcare providers about risk factors for CDI, as well as to collaborate with clinicians to ensure appropriate treatment of initial or recurrent infection based on patient-specific factors. Pharmacists are also integral to establishing and implementing antimicrobial stewardship programs that focus on appropriate antibiotic use in order to decrease the risk of CDI.
REFERENCES
1. Lawson PA, Citron DM, Tyrrell KL, Finegold SM. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe. 2016;40:95-99.
2. CDC. C. diff (Clostridioides difficile). www.cdc.gov/cdiff/index.html. Accessed September 24, 2021.
3. CDC. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019.
4. Guh AY, Mu Y, Winston LG, et al; Emerging Infections Program Clostridioides difficile Infection Working Group. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320-1330.
5. Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116(6):1124-1147.
6. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029-e1044.
7. Czepiel J, Drózdz M, Pituch H, et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38(7):1211-1221.
8. Adelman MW, Woodworth MH, Shaffer VO, et al. Critical care management of the patient with Clostridioides difficile. Crit Care Med. 2021;49(1):127-139.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery. 2017;162(2):325-348.
10. Song JH, Kim YS. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut Liver. 2019;13(1):16-24.
11. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(6):784-791.
12. Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. 2017;23(35):6500-6515.
13. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48.
14. Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67(3):742-748.
15. Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis. 2018;67(5):649-656.
16. Tsigrelis C. Recurrent Clostridioides difficile infection: recognition, management, prevention. Cleve Clin J Med. 2020;87(6):347-359.
17. Flagyl (metronidazole) package insert. New York, NY: Pfizer Inc; March 2021.
18. Dificid (fidaxomicin) package insert. Whitehouse Station, NJ: Merck & Co, Inc; February 2021.
19. Pettit NN, DePestel DD, Fohl AL, et al. Risk factors for systemic vancomycin exposure following administration of oral vancomycin for the treatment of Clostridium difficile infection. Pharmacotherapy. 2015;35(2):119-126.
20. Zinplava (bezlotoxumab) package insert. Whitehouse Station, NJ: Merck & Co, Inc; October 2016.
21. Guh AY, Kutty PK. Clostridioides difficile infection. Ann Intern Med. 2018;169(7):ITC49-ITC64.
22. Su GL, Ko CW, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159(2):697-705.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.