US Pharm. 2019;44(4):HS-9-HS-12.
ABSTRACT: Clostridium difficile is a pathogen known to cause diarrhea and colitis. If not properly treated, it can recur as well as progress to life-threatening conditions such as toxic megacolon and multiorgan failure. Guideline updates released in 2018 reflect notable changes in treatment of C difficile infection (CDI). Metronidazole is no longer recommended as first-line therapy for adults; oral vancomycin and fidaxomicin are now recommended. Current guidelines recommend fecal microbiota transplantation for patients with multiple recurrences of CDI in whom antibiotic treatment has failed. Pharmacist involvement in antibiotic stewardship programs has been shown to significantly reduce hospital rates of CDI.
Clostridium difficile, also known as C difficile, is a gram-positive, spore-forming bacteria known to cause diarrhea and colitis. Severe cases can lead to sepsis, pseudomembranous colitis, toxic megacolon, and multiorgan failure. The CDC estimates that C difficile affects a half-million people each year, and 20% of those affected may become infected again.1 It is reported that 1 in 11 people over the age of 65 years died of a healthcare-associated C difficile infection (CDI) within a month of diagnosis.1 Risk factors for C difficile include antibiotic use, age older than 65 years, recent hospitalizations, a weakened immune system, and previous CDI or known exposure.1-3
C difficile spores are spread via the fecal-oral route, hand-to-hand contact, and airborne environmental dispersal in hospitals. Symptoms of CDI usually develop shortly after antibiotic use, with risk persisting for up to 90 days.2 The highest risk of CDI occurs during and in the first month after antibiotic exposure. Extended antibiotic use and use of multiple antibiotics further increase the risk of CDI. Chemotherapy, gastrointestinal surgery, and use of acid-suppressing medications like proton pump inhibitors or histamine-2 blockers are risk factors as well.2,4
Symptoms of CDI include diarrhea with loose, watery stools, or frequent bowel movements for several days; fever; stomach tenderness or pain; loss of appetite; and nausea. Use of macrolide antibiotics including clindamycin, third and fourth generation cephalosporins, penicillins, fluoroquinolones, and carbapenems are frequently associated with CDI. Use of antibiotics suppresses normal bowel microbiota and allows C difficile to flourish.4
C difficile produces two toxins capable of causing colitis: enterotoxin (toxin A) and cytotoxin (toxin B). Toxin A is more potent. The toxins trigger neutrophils, causing inflammation of the mucosal lining, cellular necrosis, and increased peristalsis and capillary permeability, which results in diarrhea and colitis. The North American pulsed-field gel electrophoresis type 1 (NAP 1) strain of C difficile has been linked to severe outbreaks in North America and Europe. NAP 1 is reported to produce binary toxin, 16 times more toxin A, and 23 times more toxin B than other strains.5
Diagnosis of CDI
Patients with three or more unformed, unexplained, and new-onset stools in 24 hours should be tested for CDI.4 C difficile can be diagnosed by the detection of toxin A and/or toxin B in a stool sample. A stool toxin test should be used as part of a multistep algorithm with glutamate dehydrogenase (GDH) plus toxin; GDH plus toxin, arbitrated by nucleic acid amplification test (NAAT); or NAAT plus toxin, rather than NAAT alone. Enzyme immunoassay (EIA) is also used to detect toxin A or toxin B. EIAs are advantageous because they have a rapid turnaround time. GDH rapidly detects the presence of C difficile in stool samples but does not have the ability to detect production of toxins. Enzyme-linked immunosorbent assay (ELISA) tests for toxin A or toxin B also provide rapid turnaround time and high specificity.4,5
Treatment
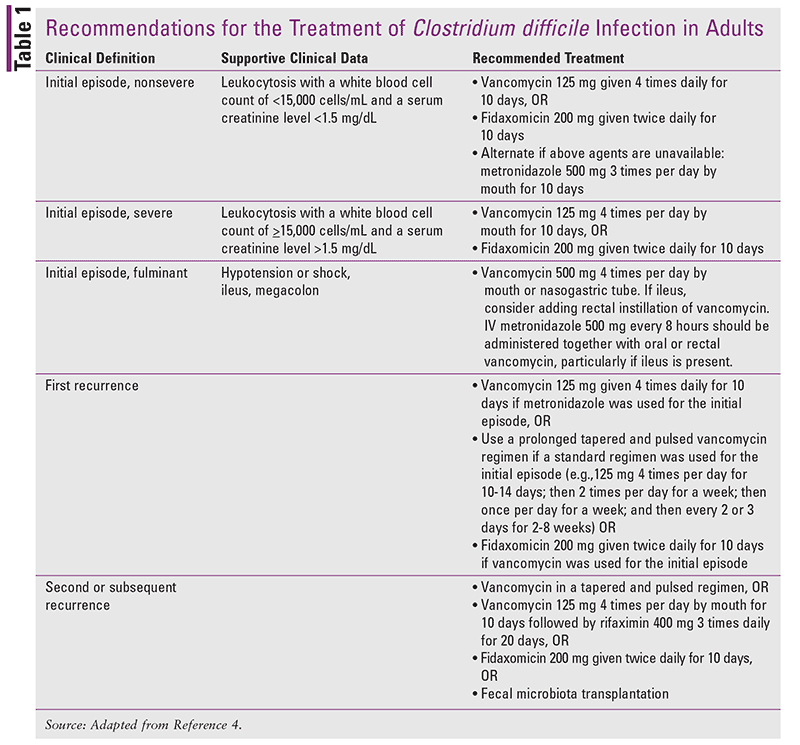
In February 2018, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) released an update in the clinical practice guidelines for CDI in adults and children, which included new recommendations.4 The biggest change from the previous guideline involves the initial treatment of CDI.
Metronidazole is no longer recommended as first-line therapy for adults. Oral vancomycin and fidaxomicin are now supported as first-line options for both non-severe and severe initial episodes of CDI. This change stems from evidence that either option ensures resolution of symptoms and sustained resolution one month after treatment. Metronidazole is only recommended for nonsevere initial episodes when patients are unable to obtain or be treated with oral vancomycin or fidaxomicin. Repeated or prolonged treatment courses should be avoided owing to the risk of neurotoxicity. Patients with fulminant CDI should receive vancomycin 500 mg 4 times per day in combination with IV metronidazole. For recurrent CDI, metronidazole should not be used. If metronidazole was used for initial treatment, patients should receive oral vancomycin. If vancomycin was used as initial treatment, vancomycin can be administered again but as a tapered and pulsed regimen, or fidaxomicin can be used.
In second or subsequent recurrences, patients can be treated with oral vancomycin, fidaxomicin, or a fecal transplant. The guideline does not advise extending CDI treatment beyond the recommended treatment course nor does it recommend restarting CDI treatment empirically for a patient who requires continued antibiotic therapy. TABLE 1 presents the treatment recommendations from the updated 2018 guidelines.4
Vancomycin
Vancomycin is a glycopeptide that inhibits bacterial cell wall synthesis by blocking glycopeptide polymerization through binding to the d-alanyl-d-alanine portion of the cell-wall precursor. Vancomycin is administered orally for treatment of CDI and is minimally systemically absorbed to achieve higher concentrations in the colon. Adverse effects of oral vancomycin include abdominal pain, dysgeusia, nausea, headache, flatulence, and peripheral edema.6,7
Fidaxomicin
Fidaxomicin (Dificid) is a macrolide antibacterial drug that is bactericidal against C difficile in vitro, inhibiting RNA synthesis by RNA polymerases; it was approved in May 2011. It is indicated for adults aged 18 years and older for the treatment of C difficile–associated diarrhea. To reduce drug-resistant bacteria and maintain effectiveness, fidaxomicin should only be used to treat infections that are either proven or strongly suspected to be caused by C difficile. The recommended dose is 200 mg orally twice daily for 10 days with or without food.
FDA approval was based on two randomized, double-blind, noninferiority trials that compared fidaxomicin with vancomycin. The primary outcomes were clinical response rate at the end of therapy based on improvement in diarrhea or other symptoms and sustained clinical response 25 days after the end of treatment. Both endpoints were achieved to show that fidaxomicin is noninferior to vancomycin. Reported adverse events include nausea, vomiting, abdominal pain, gastrointestinal hemorrhage, anemia, and neutropenia.8,9
Rifaximin
Rifaximin inhibits bacterial RNA synthesis by binding to bacterial DNA-dependent RNA polymerase. Rifaximin is recommended in the guidelines as an adjunctive post-vancomycin treatment regimen for patients with recurrent CDI. It is not absorbed and therefore has
minimal systemic effects, but there are concerns regarding potential resistance with rifaximin use. Reported common side effects include dizziness, fatigue, and nausea.4,6,10
Metronidazole
Metronidazole is a nitroimidazole that interacts with DNA to cause a loss of helical DNA structure and strand breakage resulting in inhibition of protein synthesis and cell death in susceptible organisms. Oral metronidazole is 100% bioavailable. However, there is a low concentration of medication at the site of infection due to systemic absorption, which is thought to contribute to reduced efficacy in moderate and severe cases of CDI. Reported adverse effects include headache, nausea, metallic taste, dizziness, and abdominal pain.6,11
Fecal Microbiota Transplant
The current guidelines recommend fecal microbiota transplantation for patients with multiple recurrences of CDI in whom antibiotic treatment has failed. The gastrointestinal tract is estimated to have over 160 bacterial species, with a majority residing within the colon. Since antibiotics suppress the growth of normal gut bacteria, pathogens like C difficile can proliferate. Butyrate is a short-chain fatty acid (SCFA) that is produced by bacteria that tends to be depleted in CDI. SCFA is important for energy production, immune function, and normal gut microbial growth. Recurrent CDI also reduces Bacteroidetes and Firmicutes, which are dominant gut flora. Reimplanting these strains of bacteria via fecal transplantation from healthy individuals can restore normal gut biodiversity. Mean cure rates for recurrent CDI are reported to be 91% to 96% with fecal transplantation. A variety of routes of administration have been reported in literature including nasogastric administration, rectal enema, colonoscopic administration, and oral preparations of frozen fecal microbial transplant capsules.2,4
Adjunctive Therapy
Bezlotoxumab (Zinplava), a human monoclonal antibody that binds to C difficile toxin B, was approved in October 2016. It is indicated to reduce recurrence of CDI in patients 18 years of age or older who are receiving antibacterial drug treatment for CDI and are at high risk for CDI recurrence. This medication was approved recently, after the completion of the updated guidelines, and therefore will be included in future guideline updates.12-14
Bezlotoxumab is not indicated for the treatment of CDI because it is not an antibacterial drug and should only be used in conjunction with antibacterial drug treatment. Bezlotuxumab inhibits the binding of toxin B and prevents its effects on mammalian cells. It does not bind to C difficile toxin A. The recommended dose is 10 mg/kg as an IV infusion over 60 minutes.13
FDA approval was based upon two phase 3 trials, MODIFY I and II. Both studies included more than 1,000 patients in multiple countries and were conducted in both hospital and outpatient settings. The primary outcome was evaluated through 12 weeks following study drug administration. In both MODIFY I and II, CDI recurrence was lower with bezlotoxumab than with placebo in patient groups with prior episodes of CDI, infection with the BI/NAP1/027 strain, severe CDI, age 65 years and older, and compromised immunity. Adverse effects included nausea, pyrexia, and headache. In those with a history of congestive heart failure (CHF), heart failure occurred in 12.7% of bezlotoxumab-treated patients compared with 4.8% in the placebo group. Bezlotoxumab-treated patients also had a higher mortality rate compared with placebo-treated patients. Therefore, in patients with a history of CHF, bezlotoxumab should be reserved for use when the benefits outweigh the risks.12-14
Role of the Pharmacist
One of the primary risk factors for developing CDI is antibiotic use, so pharmacists can play a vital role in minimizing patient risk through antimicrobial stewardship. Prompt initiation and administration of antibiotics have proven to reduce morbidity. However, it is estimated that 20% to 50% of all antibiotics prescribed in U.S. hospitals are unnecessary or inappropriate. Not only does inappropriate antibiotic use contribute to antibiotic resistance but it also increases potential for patient adverse events like CDI. Pharmacist involvement in antibiotic stewardship programs optimizes treatment of infections through the selection of appropriate antibiotics and de-escalation of therapy when applicable, and it has been shown to significantly reduce hospital rates of CDI.15
Pharmacists are also able to provide patient education to prevent the spread of CDI. Patients should be educated to wash their hands with soap and water every time they use the bathroom and always before eating. Anyone who cares for a patient infected with CDI should take precautions such as using gowns and gloves to prevent spread. At home, CDI patients with diarrhea should use a separate bathroom if possible. Surfaces can be cleaned with a mixture of bleach and water.16
By remaining aware of treatment updates such as those in the SHEA/IDSA 2018 guidelines, pharmacists can also assist other healthcare providers in implementing appropriate therapy for this potentially life-threatening pathogen.
REFERENCES
1. CDC. What is C. diff? Updated January 4, 2019. www.cdc.gov/cdiff/what-is.html. Accessed January 31, 2019.
2. Hopkins RJ, Wilson RB. Treatment of recurrent Clostridium difficile colitis: a narrative review. Gastroenterol Rep (Oxf). 2017;6(1):21-28.
3. Brown AWW, Wilson RB. Clostridium difficile colitis and zoonotic origins—a narrative review. Gastroenterol Rep (Oxf). 2018;6(3):157-166.
4. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis, 2018;66(7):987-994.
5. Hedge DD, Strain JD, Heins JR, Farver DK. New advances in the treatment of Clostridium difficile infection (CDI). Ther Clin Risk Manag. 2008;4(5):949-964.
6. Jarrad AM, Karoli T, Blaskovich MA, et al. Clostridium difficile drug pipeline: challenges in discovery and development of new agents. J Med Chem. 2015;58(13):5164-5185.
7. Vancomycin. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. http://online.lexi.com. Accessed February 1, 2019.
8. Dificid (fidaxomicin) package insert. Whitehouse Station, NJ: Merck & Co. Inc; 2015.
9. CenterWatch. Dificid (fidaxomicin). www.centerwatch.com/drug-information/fda-approved-drugs/drug/1152/dificid-fidaxomicin. Accessed February 1, 2019.
10. Rifaximin. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. http://online.lexi.com. Accessed February 1, 2019.
11. Metronidazole. Lexi-Drugs. Lexicomp. Riverwoods, IL: Wolters Kluwer Health, Inc. http://online.lexi.com. Accessed February 1, 2019.
12. FDA. Bezlotoxumab for injection. Drugs@FDA: FDA Approved Drug Products. March 2017. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761046. Accessed February 1, 2019.
13. Zinplava (bezlotoxumab) package insert. Whitehouse Station, NJ: Merck & Co. Inc; 2016.
14. CenterWatch. Zinplava (bezlotoxumab). www.centerwatch.com/drug-information/fda-approved-drugs/drug/100173/zinplava-bezlotoxumab. Accessed February 1, 2019.
15. CDC. Antibiotic prescribing and use in hospitals and long-term care. Core elements of hospital antibiotic stewardship programs. Updated May 7, 2015. www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html. Accessed January 31, 2019.
16. CDC. Prevent the spread of C. diff. Updated December 17, 2018. www.cdc.gov/cdiff/prevent.html. Accessed March 4, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.