US Pharm. 2022;47(7):HS-1-HS-6.
ABSTRACT: In the United States and worldwide, chronic obstructive pulmonary disease (COPD) carries a large burden of mortality and morbidity. Because the vast majority of COPD cases are due to smoking, it remains a highly preventable disease. Hallmark symptoms of COPD include dyspnea that is progressively worse, chronic cough, sputum production, and recurrent lower respiratory tract infections, and a clinical diagnosis can be confirmed with the use of spirometry measurements. Recent efforts have also focused on identifying the degree of dyspnea and impairment of quality of life when determining the optimal treatment course. Unfortunately, optimal treatment of COPD continues to be a therapeutic challenge, as current medications available for COPD largely do not modify the course of the disease. Smoking cessation in patients who are current smokers has the largest propensity to decrease disease burden and should always remain a priority for clinicians. Treatment selection is guided on severity of symptoms, spirometry measurements, and exacerbation risk.
In the United States, chronic obstructive pulmonary disease (COPD) carries a large burden of morbidity and mortality for patients. In 2020, COPD was the fifth-leading cause of death due to disease, after heart disease, cancer, death from COVID-19, and stroke.1 Unfortunately, contrary to patterns seen with other diseases, prevalence and mortality from COPD continue to climb, and death rates doubled within a span of 30 years.2,3 Because the vast majority of COPD cases are due to tobacco exposure, COPD is considered a largely preventable disease.
On a yearly basis, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines are updated. These guidelines provide a comprehensive overview of the approach to managing a patient living with COPD, including diagnosis, evaluation, and treatment strategies. In 2022, the guidelines were updated to include recommendations for COVID-19 vaccination in patients with COPD, symptoms to include nonspecific clinical manifestations such as fatigue, and evidence-based updates on traditional therapies utilized in COPD.4
Bronchitis Versus Emphysema
COPD is often viewed as an umbrella term for two main underlying diseases: emphysema and chronic bronchitis.5 Although the GOLD guidelines do not use the terms “chronic bronchitis” or “emphysema” to define COPD, it is important to understand these terms as they are often used in clinical practice to describe the pathology of COPD. Emphysema is a progressive disease caused by damage to the alveoli in the lungs. Over time, emphysema causes the walls of alveoli to become damaged and ultimately rupture. The damage to alveoli leads to larger pockets of air and a decreased surface area in the lungs, making it more difficult to breathe.6 Bronchitis is defined as inflammation of the airways, or bronchi, in the lungs. Acute bronchitis is often caused by an infection and tends to be self-limiting. Chronic bronchitis, on the other hand, develops over a much longer period of time, and symptoms never fully resolve. Chronic inflammation of the bronchi leads to an increased production of mucus in the lungs, causing breathing difficulties. Chronic bronchitis is classified by the presence of cough and sputum production for at least 3 months in each of 2 consecutive years.4,7
Risk Factors
An important component in the prevention and treatment of COPD is identifying and addressing risk factors. COPD can arise from a variety of risk factors, with the most prominent being tobacco smoking.4 In fact, 2017 data showed that adults classified as current smokers had an age-adjusted COPD prevalence of 15.2%, compared with 7.6% in former smokers and 2.8% in never-smokers.8 Smoking is correlated with a high mortality rate in COPD patients as well. Approximately 80% of the deaths associated with COPD are attributed to smoking.9 Nonetheless, 25% of patients who are diagnosed with COPD have never utilized tobacco products.10 These patients may have been exposed to other potential risk factors, including the inhalation of hazardous chemicals such as air pollutants and occupational dusts, fumes, and gases. Additionally, host-specific characteristics, such as genetic abnormalities or abnormal lung development during pregnancy and childhood, can lead to impaired lung function and increase the risk of developing COPD.4
Clinical Presentation
Hallmark symptoms of COPD include dyspnea that is progressively worse, chronic cough, sputum production, and recurrent lower respiratory tract infections.4 It should be noted, however, that patients with COPD have wide interpatient and intrapatient variability. Interestingly, some patients with severe airflow limitation may be asymptomatic.11 Therefore, a thorough clinical history of symptoms and risk factors is necessary to establish a basis for spirometry. Ultimately, the diagnosis of COPD is largely clinical in nature.
Screening and Diagnosis
Global screening for COPD is not recommended in the general population; however, a diagnosis of COPD should be considered in any patient with exposure to the risk factors and display of the hallmark symptoms mentioned previously. In patients who are suspected to have COPD, spirometry can assist in confirming a diagnosis. Spirometry can further be utilized to assist in the evaluation of severity of COPD.4
Spirometry allows for the objective measurement of airflow limitation. To obtain consistent results, spirometry should be performed after the delivery of an appropriate dose of at least one short-acting inhaled bronchodilator. As it relates to COPD, spirometry provides two key variables, forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1). FVC is a measure of the volume of air that can be forcefully exhaled following a full inhalation. FEV1 is a measure of the volume of air that can be forcefully exhaled in 1 second. The ratio of these two values (FEV1/FVC) is calculated and used to determine a diagnosis of COPD. A postbronchodilator FEV1/FVC <0.70 indicates persistent airflow limitation. Patients with exposure to previously listed risk factors, demonstrating classic COPD symptoms, and an FEV1/FVC <0.70 would meet the diagnostic criteria for COPD.4 Once a diagnosis of airflow limitation is made, it is important to ensure that the airflow limitation is irreversible, a prudent factor when distinguishing asthma from COPD.12
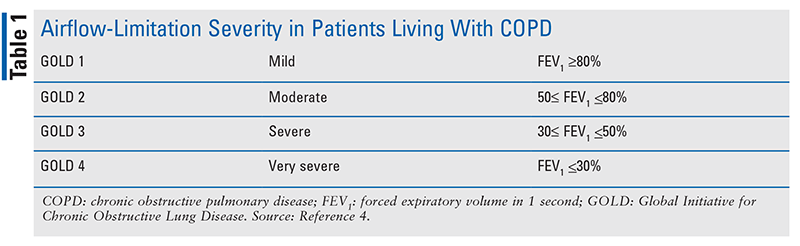
Severity of airflow limitation can also be classified based upon spirometric outcomes. TABLE 1 shows the classification of airflow limitation in patients with COPD. Of note, however, is that the severity of airflow limitation does not always correlate with the intensity or frequency of patient symptoms. Therefore, it is imperative that the assessment of a patient with COPD includes a comprehensive review of patient symptoms. Early diagnosis and treatment should be a goal, as therapies can potentially preserve and delay the progressive loss in lung functioning.4,10
A comprehensive assessment of a patient’s COPD includes more than just spirometry classifications. Once the severity of airflow limitation has been established, the severity of symptoms and risk of exacerbations should also be assessed. Recent guideline updates have shifted into a more comprehensive assessment of the impact of COPD on a patient’s overall health and quality of life. This comprehensive assessment can be achieved through completion of the Modified Medical Research Council (mMRC) Dyspnea Scale and COPD Assessment Test (CAT).4
The mMRC Dyspnea Scale rates patients on a scale of 0 to 4 based upon their degree of breathlessness (0 implies dyspnea only with strenuous exercise, while a score of 4 represents dyspnea with minimal activity).13 This, in combination with the number of exacerbations experienced by patients, can quantify symptom severity. The CAT, in contrast, can be utilized to quantify the effects of COPD on overall health status. This is an eight-question assessment that can be scored from 0 to 40, with higher scores reflecting a greater impact of COPD on everyday living.14 Cumulatively, this three-dimensional assessment can quantify severity of disease and can direct treatment approaches.
Treatment
A variety of treatment approaches can be considered in patients diagnosed with COPD and include both nonpharmacologic and pharmacologic modalities. For patients who are currently smoking, smoking cessation should be highly prioritized. In current smokers, smoking cessation has been shown to be the most effective strategy to reduce disease progression, slow residual lung decline, and decrease overall mortality.15 Nonetheless, smoking cessation can be challenging for patients to achieve. For patients who attempt to quit smoking, it is estimated that fewer than 25% will continue to be abstinent after a period of 6 to 12 months.16 What is known, however, is that behavioral interventions in combination with pharmacologic entities appear to be most effective in enhancing rates of success. A variety of pharmacologic agents are available to assist in smoking cessation, including nicotine products (lozenges, gum, patches), bupropion, and varenicline. Regardless of the treatment approach, relapse is common, and patients should be counseled that multiple attempts at smoking cessation are often required before full cessation is achieved.11
Other nonpharmacologic approaches for patients living with COPD include maintaining up-to-date influenza, pneumococcal, and TDAP vaccinations. Those who are older than age 50 years should also receive a zoster vaccine. In 2022, the GOLD guidelines were updated to include a recommendation that all patients with COPD receive vaccination against COVID-19 as well. In patients with symptomatic dyspnea, or who recently presented with an exacerbation, pulmonary rehabilitation should also be considered. Pulmonary rehabilitation has been shown to improve symptoms of dyspnea, exercise tolerance, and overall health status, and it reduces the risk of hospitalization in patients with a recent exacerbation. Studies suggest that pulmonary rehabilitation may also reduce overall anxiety and depression related to the disease process.4 However, the sustained effects of pulmonary rehabilitation appear uncertain. In one study that evaluated the sustained effects of pulmonary rehabilitation in 156 patients living with COPD, an 8-week comprehensive pulmonary rehabilitation program significantly improved dyspnea, quality of life, and anxiety and depression scores in the short term. When looking at sustained benefits, at 2 years, it appeared that the 8-week pulmonary rehabilitation program demonstrated sustained improvement in anxiety and quality-of-life scores.17
Pharmacologic Treatment Approaches
A variety of pharmacologic entities are available for the management of COPD. Pharmacologic management of COPD has been shown to reduce symptoms, reduce exacerbations, and improve overall health status. There is conflicting evidence on the benefits of COPD treatments in reducing residual lung decline, and it appears that these benefits are largely patient dependent.4 As mentioned previously, the only certain mechanism to reduce the progression of the disease and prevent disease-related mortality is to implement smoking cessation strategies in patients who are currently smoking. In terms of therapeutics, the only entity that has been shown in multiple trials to reduce mortality in patients living with COPD is long-term oxygen therapy.4
As mentioned earlier, the GOLD guidelines emphasize a comprehensive approach to assessing COPD, including severity of symptoms, risk of exacerbations, and effects on overall health status. Cumulatively, these drive management decisions.
Bronchodilators, ideally in the inhaled formulation, are the mainstay of COPD management, and they should be recommended for all patients with COPD. The use of bronchodilators has been shown to improve FEV1 and enhance symptom control.4 Short-acting bronchodilators, either in the form of a short-acting beta-2 agonist (SABA) or short-acting muscarinic antagonist (SAMA), can be utilized in patients who are only symptomatic occasionally (Group A) or for quick relief in all patients on background controller therapy.4
For patients who meet the criteria for COPD Group B, a long-acting bronchodilator is recommended. However, there are not clear guidelines for the preferred agent in this patient population. Long-acting beta-2 agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) have been shown to reduce symptoms and improve overall lung function, and patient selection should be based upon individualized response.
Of note, LAMAs appear to be superior when compared to LABAs at reducing overall exacerbation rates and therefore should be considered preferential in patients with COPD at a moderate/high risk of exacerbation and in patients with COPD Group C, who exhibit a higher baseline risk of exacerbations. If persistent dyspnea exists with monotherapy of either bronchodilator, then combination therapy with LABAs and LAMAs can also be considered as a next step. Combination therapy with a LABA and LAMA has shown to be superior to either agent used alone.4 A Cochrane review that was conducted in 2018 evaluated the safety and efficacy of various treatment approaches in COPD. They evaluated monotherapy with a LAMA or LABA and combination therapy with LAMA/LABA or LABA/inhaled corticosteroids (ICS). A total of 99 studies with over 100,000 patients were included in the analysis. Results demonstrated that symptom control and quality of life were significantly improved in combination groups when compared with monotherapy.
The greatest treatment effect, however, was seen in exacerbation reduction with LABA/LAMA combination therapy, followed by LAMA treatment. The use of LABA/LAMA in combination decreased moderate-to-severe exacerbations compared with LABA/ICS combination, LAMA monotherapy, and LABA monotherapy in the high-risk population (network hazard ratios [HRs] 0.86 [95% credible interval (CrI) 0.76 to 0.99]; 0.87 [95% CrI 0.78 to 0.99]; and 0.70 [95% CrI 0.61 to 0.8], respectively). The use of LAMA monotherapy was also shown to decrease moderate-to-severe exacerbations compared with LABA monotherapy in the high-‐and low-risk populations (network HR 0.80 [95% CrI 0.71 to 0.88] and 0.87 [95% CrI 0.78 to 0.97], respectively). LABA/LAMA combination also significantly reduced severe exacerbations when compared with LABA/ICS and LABA in the high-risk populations.18 Overall, LAMAs appear to be superior to LABAs in reducing exacerbations, as demonstrated in other clinical trials, as well.4 Combination treatment with bronchodilators of different mechanisms can provide an additive effect by sparing patients from the toxicities that occur when increasing the dose of either a beta-2 agonist or anticholinergic.
For patients with COPD Stage D, a variety of treatment regimens can be utilized. While LAMAs should be utilized in all patients classified as Stage D and are considered first line for the vast majority of patients, combination therapy with a LABA should be initiated in patients who are highly symptomatic, defined as a CAT score >20. For patients who exhibit a high risk of exacerbations, either monotherapy or combination therapy with ICS can be considered. Although considered cornerstone of care in asthma management, ICS have limited utility in patients living with COPD, and therefore, widespread use of ICS in COPD is not recommended.4
Studies have demonstrated that routine treatment with an ICS appears to increase the risk of pneumonia in patients with severe disease, rather than conferring a strong treatment benefit.4 Of note, however, is that in patients with higher baseline eosinophil counts, initial treatment is often a combination therapy of ICS/LABA, due to the decreased rate of exacerbations that occurs with this patient population.4 This benefit has been demonstrated in multiple studies, and it has been hypothesized that the degree of eosinophils can be utilized to derive treatment benefit. One meta-analysis included five studies of more than 13,000 patients with moderate-to-severe COPD. Patients with a blood eosinophil count >2% who were treated with ICS had a statistically significant reduction in exacerbations when compared with placebo treatment (risk ratio, 0.816; 95% CI, 0.67-0.99; P = .03). As was expected, there was a significantly increased risk of developing pneumonia in patients treated with an ICS. Interestingly, patients with moderate-to-severe COPD who had eosinophil counts <2% did not have significant differences in the rates of exacerbations when compared with placebo, further supporting the notion that eosinophil counts could be utilized to predict treatment benefit with ICS.19
A careful risk-versus-benefit analysis should be conducted prior to initiation of ICS. Ultimately, ICS can be considered as adjunctive treatment to one or more bronchodilators in patients with a history of frequent exacerbations that are moderate or severe in nature or in patients with evidence of asthma or elevated eosinophils.4 Special consideration for initiating ICS agents should be given to patients at a higher risk of developing pneumonia with ICS, including patients who smoke, individuals aged >55 years, low BMI, or patients presenting with severe airflow limitation.4
Triple therapy can be considered with LAMA/LABA/ICS in patients with a history of severe exacerbations. Benefits of triple therapy in reducing exacerbations were demonstrated in the ETHOS trial. The ETHOS trial was a randomized, controlled trial of more than 8,000 patients with moderate-to-severe COPD. Patients who had at least one exacerbation in the past year were randomized to one of four treatment groups that included both triple- and dual-therapy regimens. Treatment groups included 1) twice-daily inhaled doses of triple therapy at two different doses (inhaled glucocorticoid [320 µg or 160 µg of budesonide], a LAMA [18 µg of glycopyrrolate], and a LABA [9.6 µg of formoterol]); 2) LAMA + LABA (18 µg of glycopyrrolate plus 9.6 µg of formoterol); or 3) ICS (320 µg of budesonide) + LABA (9.6 µg of formoterol). The primary endpoint was the annual rate (the estimated mean number per patient per year) of moderate-or-severe COPD exacerbations.
At the conclusion of the 52-week treatment period, patients who were treated with triple therapy, in both doses, had a significantly lower rate of COPD exacerbations when compared with either arm of dual therapy. As expected, the rate of pneumonia in patients treated with ICS was higher than seen without this treatment component. Nonetheless, the study demonstrated that triple therapy could reduce exacerbation rate in patients at a high risk with moderate-to-severe COPD.20 In a further analysis of the ETHOS trial, there appears to be a mortality benefit in patients with triple therapy as well. High-dose ICS triple therapy was associated with a significantly lower risk of death when compared to LAMA/LABA dual therapy (HR, 0.51; 95% CI, .33-0.80; unadjusted P = .0035). No mortality benefit was conferred when comparing ICS triple therapy with ICS/LABA therapy or with the lower doses of ICS as triple therapy.21 However, adverse effects of ICS appear to be dose dependent, so a careful analysis of who to initiate therapy in is important prior to initiating high-dose ICS.
Conclusion
COPD is a lung disease characterized by a progressively worsening lung function and increased difficulty in breathing. COPD can be diagnosed and classified based on spirometry results as well as the frequency and severity of hallmark symptoms and exacerbations. The most beneficial method to slow the progression of COPD and decrease mortality is to institute adequate smoking cessation therapy in patients who are current smokers. For patients requiring pharmacologic therapy to treat COPD, inhaled bronchodilators remain the cornerstone of therapy, and monotherapy with a LAMA or LABA may be sufficient at reducing and preventing symptoms in some patients. Inhaled corticosteroids can be considered in specific populations, including those at a high risk of exacerbations or elevated baseline eopsinophil counts, although combination regimens are associated with an increased risk of adverse effects. Ultimately, selection of drug therapy should consider patient-specific factors and preferences as well as an assessment of the risks and benefits of each medication.
REFERENCES
1. CDC. Leading Causes of Heath. www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed May 1, 2022.
2. Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(5 suppl):121S-126S.
3. Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255-1259.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease – 2022 report. November 2021.
5. American Lung Association. Chronic Obstructive Pulmonary Disease. www.lung.org/lung-health-diseases/lung-disease-lookup/copd. Accessed April 28, 2022.
6. American Lung Association. Emphysema. www.lung.org/lung-health diseases/lung-disease-lookup/emphysema. Accessed April 28, 2022.
7. American Lung Association. Bronchitis. www.lung.org/lung-health-diseases/lung-disease-lookup/chronic-bronchitis. Accessed April 28, 2022.
8. Wheaton AG, Liu Y, Croft JB, et al. Chronic obstructive pulmonary disease and smoking status — United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:533-538.
9. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Accessed April 28, 2022.
10. CDC. Chronic Obstructive Pulmonary Disease and Smoking Status — United States, 2017. MMWR Morb Mortal Wkly Rep. June 21, 2019. Accessed April 8, 2022.
11. Devine, J. HDB. 2008;1(7):34-42.
12. Rogliani P, Ora J, Puxeddu E, Cazzola M. Airflow obstruction: is it asthma or is it COPD?. Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
13. mMRC (Modified Medical Research Council) Dyspnea Scale – MDCalc. Accessed May 6, 2022.
14. American Thoracic Society. COPD Assessment Test(CAT). www.thoracic.org/members/assemblies/assemblies/srn/questionaires/copd.php. Accessed May 6, 2022.
15. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 145-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233-239.
16. Tashkin, D.P. Smoking cessation in COPD: confronting the challenge. Intern Emerg Med. 2021;16:545-547.
17. Yohannes AM, Dryden S, Casaburi R, et al. Long-term benefits of pulmonary rehabilitation in patients with COPD: a 2-year follow-up study. Chest. 2021;159(3):967-974.
18. Oba Y, Keeney E, Ghatehorde N, et al. Dual combination therapy versus long‐acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews. 2018;12:CD012620.
19. Cheng, Shih-Lung. Blood eosinophils and inhaled corticosteroids in patients with COPD: systematic review and meta-analysis. International Journal of Chronic Obstructive Pulmonary Disease. 2018;13:2775-2784.
20. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35-48.
21. Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203(5):553-564.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






