US Pharm.
2008;33(7):36-43.
Pneumococcal infections cause
an estimated 3,000 cases of meningitis, 50,000 cases of bacteremia, and
500,000 cases of pneumonia annually.1 Centers for Disease Control
and Prevention (CDC) surveillance data suggest that persons younger than 2
years of age and those 65 years and older account for the highest percentage
of cases. Consequently, one goal of the Healthy People 2010 initiative is to
decrease the incidence of invasive pneumococcal infections to 46 per 100,000
persons younger than 5 years and 42 per 100,000 persons aged 65 and older.
2 Vaccination appears to be the most effective strategy for preventing
invasive pneumococcal disease (IPD), especially since treatment is hampered by
the increasing resistance to available antibiotics.
Background
For more than a
century, the study of Streptococcus pneumoniae and pneumococcal
infections has occupied a central position in the development of a scientific
basis for the control of infectious diseases. The organism was first isolated
almost simultaneously by both Sternberg and Pasteur in 1881. In the
early 1960s, Austrian and Gold demonstrated that pneumococcal infections
remained lethal despite appropriate antimicrobial therapy.3,4
The major clinical syndromes
caused by S pneumoniae are widely recognized. Direct spread of the
organism from the nasopharynx leads to otitis media, sinusitis, and pneumonia.
Invasive pneumococcal disease is defined by the detection of S pneumoniae
in the bloodstream; the presence of primary bacteremia; or hematogenous
spread of the organism, causing meningitis and endocarditis.3,4
The Organism
Pneumococci are
facultative anaerobes that grow in short chains and appear as Gram-positive
diplococci. Cell-wall polysaccharide is unique to S pneumoniae and
consists of a peptidoglycan backbone, which plays a major role in stimulating
the inflammatory response associated with pneumococcal infections. The
capsular polysaccharide is one of the primary factors responsible for the
virulence of the organism. Unlike peptidoglycan, capsular polysaccharide does
not induce an inflammatory response, but inhibits phagocytosis and interferes
with intracellular killing of phagocytized pneumococci.3
Differences in the chemical
structures of pneumococcal capsular polysaccharides provide the basis for
classifying the organism, and 90 different serotypes have been identified to
date.3,4 Fortunately, only a limited number of serotypes
account for most pneumococcal disease in humans. The seven most common
serotypes account for roughly 80% of infections in children aged 6 years and
younger; this drops to 50% in older children and adults, illustrating the
variances in epidemiology of individual serotypes among different age groups.
4
Pathogenesis
S pneumoniae
colonizes the nasopharynx, and culturing yields pneumococci in 5% to 10% of
healthy adults and 20% to 40% of healthy children. The overall rate of
IPD in blood, pleural fluid, or cerebrospinal fluid (CSF) is approximately 15
per 100,000 persons annually. Transmission of S pneumoniae occurs as a
result of direct person-to-person contact by respiratory droplets and
autoinoculation in persons carrying the pneumococci in their upper respiratory
tract.5
Several nonimmunologic and
immunologic factors act together to defend the host against pneumococcal
infection. Nonimmunologic factors include gag and cough reflexes, as well as
ciliary clearance mechanisms in the bronchial tree. Immunologic factors
include phagocytic cells and sufficient concentrations of antibody and
complement. A deficiency in any of these mechanisms can be implicated in most
cases of pneumococcal infection.3
Suppression of the gag or
cough reflex can be induced by alcohol, opiates, or aging.3
Cigarette smoking is the strongest independent risk factor for IPD among
immunocompetent, nonelderly adults.6 Crowding in such varied
environments as day-care centers, homeless shelters, and prisons increases the
risk of exposure. Alcohol ingestion, renal or hepatic insufficiency,
glucocorticoid treatment, and diabetes mellitus adversely affect the migration
of and bacterial killing by polymorphonuclear leukocytes.3
The spleen is the principal
organ that clears pneumococci; consequently, infection occurs overwhelmingly
in children and adults whose spleen has been removed or is dysfunctional.
Defective antibody formation has the greatest impact on susceptibility to
pneumococcal infection. Other factors that predispose to IPD are: CSF
leaks; chronic lung disease; congenital immune deficiency; congestive heart
failure; immunosuppression; HIV infection; malignancy; and sickle cell
disease. Children aged 24 to 35 months and those aged 36 to 59 months who are
of Native American or African American descent also are susceptible to IPD.
5,7
Clinical Manifestations
Pneumococcal
pneumonia is the most common clinical presentation among adults, especially
those aged 65 years and older. Approximately 175,000 hospitalizations occur
annually in the United States, and pneumococci account for up to 36% of adult
community-acquired pneumonia (CAP) cases and 50% of hospital-acquired
pneumonia cases.4
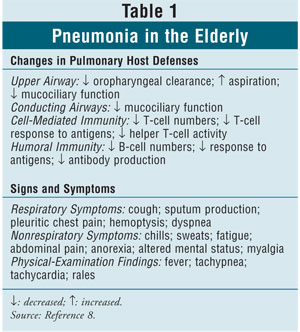
Age-related changes in
pulmonary host defenses and signs and symptoms of pneumonia in the elderly
appear in TABLE 1.8 Although elderly persons may show
fewer signs, symptoms, and physical findings than younger adults, 60% of both
age groups manifest a classic bacterial pneumonia syndrome, defined as having
at least three of the following signs and symptoms: acute onset, chills, chest
pain, and purulent sputum.8,9
Pneumococcal meningitis, with or
without accompanying bacteremia, is the next most common form of invasive
disease.3 Since bacterial meningitis due to Haemophilus
influenzae type B was virtually eliminated through routine vaccination,
pneumococcal meningitis has become the leading cause in both adults and
children, with a mortality rate ranging from 16% to 37% and neurologic
complications including coma, focal neurologic deficits, and seizures in 30%
to 52% of surviving adults.10,11 Most patients have predisposing
factors similar to those seen in pneumonia, such as existing infection or
immunocompromised state.10 Persons with cochlear implants
also appear to be at increased risk.4
At least two of the following
classic symptoms are seen in most patients with pneumococcal meningitis:
headache, fever, stiff neck, and altered mental status. Increased severity is
indicated by the presence of neurologic complications on admission.10,11
Despite treatment, most patients develop complications, including
cerebrovascular sequelae, cranial nerve palsies, septic shock,
cardiorespiratory failure, and disseminated intravascular coagulation.
10,11
Diagnosis
A pneumococcal
infection is diagnosed when S pneumoniae is cultured from the blood or
a normally sterile extrapulmonary site such as the CSF, pleural fluid, or
synovial fluid. The quellung reaction is a test that provides rapid
identification of pneumococci in clinical specimens such as CSF, sputum, and
exudates. If the test is positive, a large capsule surrounds the organism.
4
Microscopic examination and
culture of sputum samples is a potentially useful diagnostic test for
pneumonia. However, 30% to 40% of patients fail to produce sputum and may have
received prior antibiotic therapy.3 A promising approach has been
developed that uses enzyme-linked immunosorbent assay to detect increases in
antibodies to pneumococcal surface antigen A in the sputum and urine.3
Chest radiography reveals an area of infiltration involving a single lobe.
8
Lumbar puncture is essential
in the diagnosis of pneumococcal meningitis. Gram staining and culturing of
CSF can identify pneumococci. Cranial imaging shows hypodense lesions, brain
swelling, and hydrocephalus.10
Considerable attention has
been given to improving the accuracy of tests that diagnose pneumococcal
infections by detecting pneumococcal antigen in the sputum, blood, CSF, or
urine. Unfortunately, most of the newer techniques have not added
substantially to the diagnostic utility of bacteriologic methods alone.3
Treatment
The basic
principles of treatment for pneumococcal infection are similar to those for
treating other types of infection.5 Treatment generally includes
antibiotics that are effective against S pneumoniae and other potential
pathogens since the causative organism is not known at the time treatment is
started.
Patients with pneumococcal
pneumonia are considered for outpatient versus inpatient treatment based on
age, severity of illness, and comorbidities. Treatment with third-generation
cephalosporins, quinolones, macrolides, penicillins (with or without
beta-lactamase inhibitors), and doxycycline all seem to be equally effective.
Duration of therapy is usually 10 to 14 days.8,9
Standard agents used to treat
pneumococcal meningitis are penicillin, ampicillin, cefotaxime, ceftriaxone,
and vancomycin. Dexamethasone is the only accepted adjunctive therapy;
it has been shown to decrease mortality and neurologic complications.10,11
Antibiotic Resistance and
Vaccination
The increasing
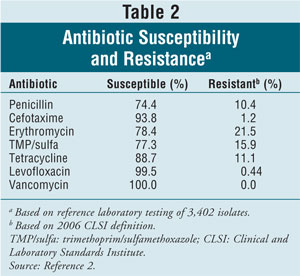
resistance of pneumococci to commonly used antibiotics has made treatment
decisions more difficult. Susceptibility and resistance rates for common
antibiotics used to treat invasive pneumococcal infections are given in
TABLE 2.2 Fortunately, the seven serotypes in the
pneumococcal conjugate vaccine account for most drug-resistant S pneumoniae
, leading to a decrease in rates of antibiotic-resistant invasive pneumococcal
infections in both children and the elderly.12-15
Vaccination remains the
primary preventative strategy for CAP in the elderly.8 Routine
immunization with the polysaccharide conjugate vaccine has reduced the risk of
invasive S pneumoniae infection in young children by 90% and indirectly
decreased the incidence in older adults.15,16 Despite CDC
recommendations, most patients, especially elderly ones, do not obtain the
appropriate vaccinations. In 2002, a study of patients' knowledge and
perceptions regarding vaccinations found that patients who were not up-to-date
with their pneumococcal vaccinations were unaware that they were at risk and
did not recognize the need for the vaccine.17
Vaccines
Pneumococcal
Polysaccharide Vaccine (PPV23):
PPV23 comprises purified
preparations of polysaccharide capsular antigen from 23 types of pneumococcal
bacteria that cause 88% of bacteremic pneumococcal disease. The vaccine
currently available in the U.S., Pneumovax 23, contains 25 mcg of each antigen
per dose and 0.25% phenol as a preservative. It is available as a single-dose
vial or syringe and as a five-dose vial.4,18
More than 80% of healthy
adults who receive PPV23 develop antibodies against the serotypes contained in
the vaccine, usually within two to three weeks after vaccination. Elevated
antibody levels persist for at least five years in healthy adults, but decline
more quickly in persons with underlying illnesses. Overall, the vaccine is 60%
to 70% effective in preventing invasive disease, specifically bacteremia, but
has not been shown to provide protection against pneumococcal pneumonia.
4,18,19
Because the target groups for
the PPV23 and influenza vaccines overlap, these two vaccines can be given
concurrently. In addition to adults aged 65 years and older, immunocompromised
persons aged 2 or more years who are at increased risk for pneumococcal
disease or its complications should be vaccinated. Those with chronic illness
and those who are in a high-risk environment (e.g., institution) also should
be vaccinated. If elective splenectomy or cochlear implantation is being
considered, the vaccine should be given at least two weeks before, or as soon
as possible after, the procedure.4,18,20
Most recipients will need only
a single lifetime dose of PPV23. Following vaccination, antibody levels
decline after five to 10 years, but a higher antibody level does not
necessarily correlate with better protection. Routine PPV23 revaccination of
immunocompetent persons is not recommended.4,18,19
For high-risk patients, only
one revaccination dose is recommended. The second dose should be administered
at least five years after the first dose. Revaccination three years after the
first dose may be considered for children at high risk for severe pneumococcal
infection who are no older than 10 years at the time of revaccination. Persons
aged 65 years and older should be administered a second dose of pneumococcal
vaccine if they received the first dose more than five years previously and
were younger than 65 years at the time.4,19,21
Pneumococcal Conjugate
Vaccine (PCV7):
The first pneumococcal conjugate vaccine, Prevnar, was licensed in the U.S. in
2000. It comprises purified capsular polysaccharide of seven serotypes of S
pneumoniae conjugated to a nontoxic variant of diphtheria toxin. After
four doses, more than 90% of healthy infants develop antibodies to all seven
serotypes. PCV7 was shown to reduce invasive disease caused by vaccine
serotypes by 97%, and disease caused by all serotypes--including those not in
the vaccine--by 89%.4,7 All children younger than 24
months of age and those aged 24 to 59 months with a high-risk medical
condition should be routinely vaccinated with PCV7. The primary series
consists of three doses given at 2, 4, and 6 months of age. The fourth booster
dose is recommended at 12 to 15 months of age. Unvaccinated children aged 7
months or older require fewer doses. For children vaccinated before 12 months
of age, the minimum interval between doses is four weeks. Doses given at age
12 months and older should be separated by at least eight weeks. PCV7 is not
routinely recommended for children older than 59 months; revaccination with
PCV7 after an age-appropriate primary series also is not currently recommended.
4,7,22,23
Adverse Reactions and
Contraindications:
Both vaccines are inactive and administered by intramuscular injection;
therefore, the most common adverse reactions are local site reactions. Pain,
swelling, and erythema have been reported and persist for less than 48 hours.
The incidence is 30% to 50% for PPV23 and 10% to 20% for PCV7.4,7,18
A severe allergic reaction to
a vaccine component or following a prior dose is a contraindication to
administering further doses. Patients with moderate or severe acute illness
should wait until their condition improves before being vaccinated. Contrary
to popular belief, minor illnesses such as upper respiratory infections are
not a contraindication to vaccination. The safety of PPV23 vaccine in pregnant
women has not been studied, although no adverse consequences have been
reported.4,7,18
Storage and Handling:
Store PPV23 and PCV7
under refrigeration (35°F-46°F [2°C-8°C]). Do not freeze pneumococcal
vaccines. Opened multidose vials may be used until the expiration date if they
are not visibly contaminated.4,7,18
Conclusion
One of the Healthy
People 2010 objectives is to achieve 90% coverage of noninstitutionalized
adults aged 65 years and older for pneumococcal polysaccharide vaccine.
According to data from the 2005 Behavioral Risk Factor Surveillance System
surveys, the overall proportion of respondents who reported ever having
received pneumococcal polysaccharide vaccine was approximately 64%.24
The pharmacist is in an
opportune position to help improve PPV23 adult vaccination rates. The
pharmacist's role can involve asking older patients about their
pneumococcal-vaccination history; educating patients about the benefits of
pneumococcal vaccination; reviewing patient charts or prescription records to
identify targeted chronic diseases; and vaccinating appropriate at-risk
patients (if permitted by law in that state). Through the screening and
vaccination of individuals found to be at high risk, the pharmacist can help
significantly reduce the complications and mortality associated with
pneumococcal disease.
REFERENCES
1. Improving
influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage
among adults aged <65 years at high risk. A report on recommendations of the
Task Force on Community Preventive Services. MMWR. 2005;54:1-12.
2. Centers for Disease
Control and Prevention. Active Bacterial Core (ABC) surveillance report,
Emerging Infections Program Network, Streptococcus pneumoniae, 2006.
www.cdc.gov/ncidod/dbmd/abcs/survreports/spneu06.pdf. Accessed April 7, 2008.
3. Plotkin SA,
Orenstein WA. Vaccines. 4th ed. Philadelphia, PA: Elsevier, Inc; 2004.
4. Atkinson W,
Hamborsky J, McIntyre L, et al, eds. Epidemiology and Prevention of
Vaccine-Preventable Diseases. 10th ed. Washington, DC: Public Health
Foundation; 2008.
5. Musher DM.
Streptococcus pneumoniae. In: Mandell GL, Bennett JE, Dolin R, eds.
Mandell, Douglas, & Bennett's Principles and Practice of Infectious Diseases.
6th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005.
6. Nuorti JP, Butler
JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease.
N Engl J Med.2000;342:681-689.
7. Prevnar
(pneumococcal 7-valent conjugate vaccine [diphtheria CRM197
protein]) package insert. Philadelphia, PA: Wyeth Pharmaceuticals Inc;
December 2007.
8. Donowitz GR, Cox HL.
Bacterial community-acquired pneumonia in older patients. Clin
Geriatr Med.2007;23:515-534.
9. Halm EA, Teirstein
AS. Clinical practice. Management of community-acquired pneumonia. N Engl J
Med. 2002;347:2039-2045.
10. Weisfelt M, de Gans
J, van der Poll T, van de Beek D. Pneumococcal meningitis in adults: new
approaches to management and prevention. Lancet Neurol. 2006;5:332-342.
11. van de Beek D, de
Gans J, Tunkel AR, Wijdicks EFM. Community-acquired bacterial meningitis in
adults. N Engl J Med.2006;354:44-53.
12. Centers for Disease
Control and Prevention Division of Bacterial and Mycotic Diseases.
Drug-resistant Streptococcus pneumoniae disease. 2005. Available at:
www.cdc.gov/ncidod/dbmd/diseaseinfo/drugresisstreppneum_t.htm. Accessed April
7, 2008.
13. Whitney CG, Farley
MM, Hadler J, et al. Increasing prevalence of multidrug-resistant
Streptococcus pneumoniae in the United States. N Eng J Med.
2000;343:1917-1924.
14. Kyaw MH, Lynfield
R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate
vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med.
2006;354:1455-1463.
15. Lexau CA, Lynfield
R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease
among older adults in the era of pediatric pneumococcal conjugate vaccine.
JAMA. 2005;294:2043-2051.
16. Carstairs KL, Tanen
DA, Johnson AS, et al. Pneumococcal bacteremia in febrile infants presenting
to the emergency department before and after the introduction of the
heptavalent pneumococcal vaccine. Ann Emerg Med. 2007;49:772-777.
17. Santibanez TA,
Nowalk MP, Zimmerman RK, et al. Knowledge and beliefs about influenza,
pneumococcal disease, and immunizations among older people. J Am Geriatr
Soc. 2002;50:1711-1716.
18. Pneumovax 23
(pneumococcal vaccine polyvalent) package insert. Whitehouse Station, NJ:
Merck & Co, Inc; January 2008.
19. Whitney CG.
Preventing pneumococcal disease: ACIP recommends pneumococcal polysaccharide
vaccine for all adults age ?65. Geriatrics. 2003;58:20-2,25.
20. Jackson LA, Neuzil
KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older
adults. N Engl J Med.2003;348:1747-1755.
21. Prevention of
pneumococcal disease. Recommendations of the Advisory Committee on
Immunization Practices (ACIP). MMWR. 1997;46:1-24.
22. American Academy of
Pediatrics. Policy statement: recommendations for the prevention of
pneumococcal infections, including the use of pneumococcal conjugate vaccine
(Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis.
Pediatrics. 2000;106(2):362-366.
23. Preventing
pneumococcal disease among infants and young children: recommendations of the
Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep.
2000;49:1-35.
24. Influenza and
pneumococcal vaccination coverage among persons aged ?65 years--United
States, 2004-2005. MMWR. 2006;55:1065-1068.





