US Pharm. 2016;41(11):43-44.
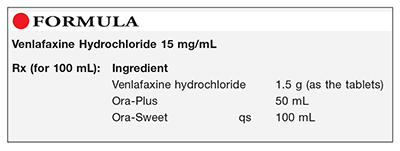
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient or obtain the required quantity. Thoroughly pulverize the venlafaxine hydrochloride tablets to a fine powder. Add a few mL of Ora-Plus to the powder and mix to form a smooth paste. Geometrically, incorporate the remainder of the Ora-Plus and mix well. Add sufficient Ora-Sweet to final volume and mix well. Package and label.
Note: As an alternative, simple syrup may be used as the vehicle, replacing the Ora-Plus and Ora-Sweet, with the same stability.
Use: Venlafaxine hydrochloride oral suspension has been used in the treatment of major depressive disorder, anxiety, and panic disorder.
Packaging: Package in tight containers.
Labeling: Keep out of reach of children. Keep refrigerated. Shake well. Discard after ____ [time period].
Stability: A beyond-use date of up to 28 days at either room temperature or refrigerated temperature may be used for this preparation.1,2
Quality Control: Quality-control assessment may include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, and mold growth).3
Discussion: Venlafaxine hydrochloride (Effexor, C17H27NO2.HCl, MW 313.86) occurs as an off-white to white, crystalline powder that is soluble 572 mg/mL in water and melts at about 217°C. It is available as both immediate-release and extended-release tablets. Either dosage form may be used for this preparation as long as the tablets are thoroughly pulverized to disrupt the coatings completely. Drug release is controlled by diffusion through the coating membrane on the spheroids and is not pH-dependent. Venlafaxine capsules also contain cellulose, ethylcellulose, gelatin, hypromellose, iron oxide, and titanium dioxide.4
Ora-Plus is an oral suspending vehicle that accepts dilution of up to 50% or more with water, flavoring agents, or syrups while still retaining its suspending properties. Ora-Plus has a pH of approximately 4.2 and an osmolality of about 230 mOsm/kg. It is a thixotropic vehicle with a viscosity of approximately 1,000 cps at 25°C. Ora-Plus contains purified water, microcrystalline cellulose, sodium carboxymethylcellulose, xanthan gum, carrageenan, sodium phosphate, and citric acid as buffering agents; simethicone as an antifoaming agent; and potassium sorbate and methylparaben as preservatives.5
Ora-Sweet syrup vehicle is a flavoring vehicle for oral extemporaneous preparations. It is flavored with a citrus-berry flavor blend and contains glycerin and sorbitol to prevent cap lock, a problem associated with many syrups. Ora-Sweet is buffered to a pH of approximately 4.2, and it has an osmolality of about 3,240 mOsm/kg. Ora-Sweet contains purified water, sucrose, glycerin, sorbitol (5%), flavoring, sodium phosphate, and citric acid as buffering agents and potassium sorbate and methylparaben as preservatives.6
Syrup (simple syrup) is a clear, sweet vehicle used as a sweetening agent and as the base for many flavored and medicated syrups. It contains 85% w/v sucrose in water and has a specific gravity of not less than 1.30. Syrup is generally self-preserving as long as the sucrose concentration is maintained sufficiently high. It is preferred that syrup be prepared without the use of heat, but it may be prepared by the use of boiling water. Syrup should be stored in tight containers, preferably in a cool place.1
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; October 2016.
2. Donnelly RF, Wong K, Goddard R, Johanson C. Stability of venlafaxine immediate-release suspensions. Int J Pharm Compd. 2011;15:81-84.
3. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
4. RxList. Effexor XR. www.rxlist.com/effexor-xr-drug.htm. Accessed October 13, 2016.
5. Ora-Plus product information. Allegan, MI: Perrigo; 2014.
6. Ora-Sweet product information. Allegan, MI: Perrigo; 2014.
To comment on this article, contact rdavidson@uspharmacist.com.





