US Pharm. 2023;48(6):HS8-HS12.
ABSTRACT: Heart failure continues to be a leading cause of morbidity and mortality in the United States. Due to this, new medications are being investigated to reduce morbidity and mortality in this patient population. Vericiguat presents a new option for the treatment of heart failure with reduced ejection fraction. Patients most likely to benefit from the addition of vericiguat are those who are aged younger than 75 years, have moderately reduced kidney function, are New York Heart Association class III or IV, or are unable to tolerate an angiotensin receptor/neprilysin inhibitor.
Heart failure (HF) is a complex syndrome caused by structural and functional impairments in the myocardium resulting in impairment of ventricular filling or the ejection of blood.1 From the first trials demonstrating mortality benefits with the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACE/ARBs), mineralocorticoid receptor antagonists (MRAs), and beta blockers (BBs) in late 1990s to early 2000s, very little has changed in the treatment of HF with reduced ejection fraction (HFrEF).2-5 In 2014, the PARADIGM-HF trial demonstrated that the use of sacubitril/valsartan has a greater reduction in cardiovascular (CV) mortality and HF hospitalizations compared with enalapril.6 This led to the guideline updates in 2017 and 2021 that recommended the use of sacubitril/valsartan as an alternative to ACE/ARBs and eventually recommended sacubitril/valsartan over ACE/ARBs.7,8 In 2020, the empagliflozin outcome in patients with chronic HFrEF (EMPEROR-Reduced) and dapagliflozin on the incidence of worsening HF or CV death in patients with chronic HF (DAPA-HF) trials demonstrated a reduction in mortality and HF hospitalization with the use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) regardless of diabetes.9,10 These trials gained SGLT2i a class I recommendation in the 2021 HF update.8
Also published in 2020 was the VICTORIA trial, which presents another option, vericiguat, for the treatment of patients with HFrEF.11 Vericiguat is a soluble guanylate cyclase agonist. The vascular endothelium normally generates nitric oxide (NO), which stimulates soluble guanylate cyclase (sGC)–mediated cyclic guanosine monophosphate (cGMP) production, leading to vasodilatation. This process becomes dysregulated in HF, leading to NO-sGC-cGMP insufficiency. Vericiguat modulates sGC and enhances the effect of NO to increase cGMP activity.11
THE VICTORIA TRIAL
Rationale
The rationale for the VICTORIA trial was established in the SOCRATES-Reduced randomized phase IIb dose-finding study.12 This study failed to show significance in its prespecified composite endpoint that pooled the three highest vericiguat doses; however, it did note numerical trends in CV deaths and hospitalization along with improved left ventricular ejection fraction (EF) in the group on the highest dose. This earlier trial also established hypotension and syncope as adverse events of clinical interest.
Trial Design
The VICTORIA trial was a randomized, placebo-controlled, parallel-group, multicenter, double-blind, event-driven trial for subjects with HFrEF. The primary objective was to test whether vericiguat decreased the time to the first occurrence of CV death or HF hospitalization in patients with HFrEF versus placebo. Secondary objectives evaluated each component of the primary outcome and key adverse effects. After a 30-day screening process, 5,050 patients were randomized 1:1 into vericiguat (n = 2,526) and placebo (n = 2,524) groups.
Inclusion Criteria
The study population consisted of chronic HF patients aged 18 years old or older with New York Heart Association (NYHA) functional class II to IV with a reduced EF of <45% within 12 months prior to randomization and elevated natriuretic peptide levels within 30 days of randomization.
The qualifying event had to have occurred within 6 months of randomization and included hospitalization or within 3 months of randomization and received IV diuretic without hospitalization (subjects whose event occurred 3-6 months before randomization were limited to 20%).
Exclusion Criteria
Clinically unstable patients who received IV treatment 24 hours before randomization and/or had a systolic blood pressure <100 mmHg or symptomatic hypotension were excluded. Patients taking long-acting nitrates or NO donors were excluded. Subjects taking phosphodiesterase type 5 or another sGC stimulator were excluded. Anyone with known allergies or sensitivities to sGC stimulators or any components of the investigational product were excluded from the trial. HF patients on a continuous infusion of an inotrope, with an implanted ventricular assist device, or having other cardiac comorbidities were excluded from this trial. Patients awaiting heart transplantation were excluded. Other exclusions included patients on chronic dialysis, with severe hepatic insufficiency, or with pulmonary disease, and pregnant or breastfeeding women.
Intervention/Follow-up
After randomization, patients were initiated with a 2.5-mg dose of vericiguat or matching placebo. The doses were titrated up from 2.5 mg to 5 mg to 10 mg. The researchers evaluated patients at 2 and 4 weeks, and then every 4 months, until a planned number of events was reached. Fourteen days after the final visit, subjects received a final phone call to wrap up the study. At each evaluation, researchers addressed dosing to increase the likelihood of achieving and maintaining the 10-mg target dose. See FIGURE 1 for the titration schedule.
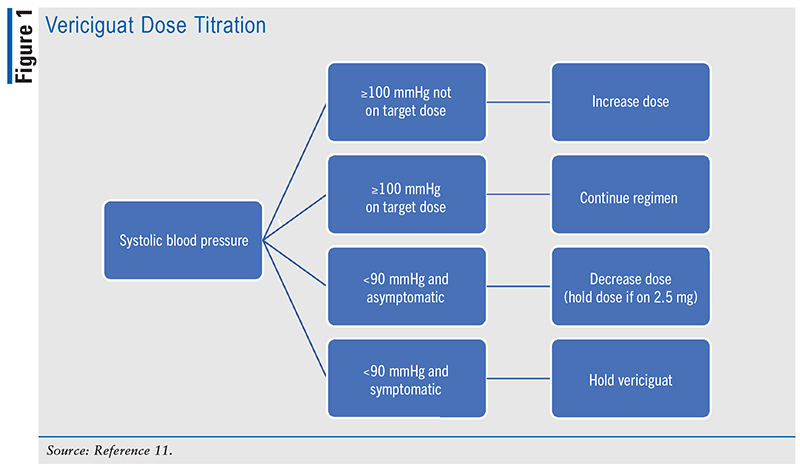
Ascertainment for the primary outcome was completed for 99.5% and 99.6% of vericiguat and placebo, respectively. The median follow-up was 10.8 months.
VERICIGUAT IN THE 2022 HEART FAILURE GUIDELINES
Despite the results of the VICTORIA trial, the 2021 guideline update only briefly mentioned the use of vericiguat as an emerging treatment option. The most recent HF guidelines, published in 2022, stated that for patient on guideline-directed medical therapy (including treatment with an ACE, ARB, angiotensin receptor/neprilysin inhibitor [ARNI], BB, SGLT2, or MRA) whose EF remains <45%, the addition of vericiguat may be considered. Although these guidelines do mention vericiguat more than the 2021 update, there is still confusion when to initiate this medication. By taking a deeper look at the subgroup analysis of the VICTORIA trial, a better determination of which patients are most likely to benefit from the addition of vericiguat is possible.
SUBGROUP ANALYSIS OF THE VICTORIA TRIAL
The subgroup analysis of the VICTORIA trial revealed a few populations more likely to benefit from the addition of vericiguat. The subgroup analysis demonstrates that the vericiguat is more effective in patients aged 75 years or younger. For patients aged younger than 75 years, the primary outcome occurred in 579 patients in the vericiguat group and 669 patients in the placebo group (hazard ratio [HR] 0.84, CI 0.75-0.94). In patients aged older than 75 years, the primary outcome occurred in 318 patients in the vericiguat group and 303 patients in the placebo group (HR 1.04, CI 0.88-1.2).
The N-terminal pro–B-type natriuretic peptide (NT-proBNP) level is clinically helpful for diagnosing or excluding and predicting the prognosis and adverse outcomes of patients with congestive HF. The subgroup analysis of the VICTORIA trial revealed that vericiguat use reduced the incidence of primary outcomes more among the patients with NT-proBNP ≤5,314.0 pg/mL than the patients in the NT-proBNP >5,314.0 pg/mL group.
This analysis showed that vericiguat was more effective at improving and reducing the primary outcome in patients with NYHA class III-IV than patients with NYHA class I-II. For patients with NYHA class III-IV, the primary outcome occurred in 451 patients in the vericiguat group and 487 patients in the placebo group (HR 0.87, CI 0.77-0.99). Compared with patients classified NYHA I-II, the primary outcome occurred in 445 patients in the vericiguat group and 484 patients in placebo group (HR 0.91, nonsignificant CI 0.80-1.04).
There was a trend toward the decreased risk of CV death or HF hospitalization in patients who are not on concomitant use of sacubitril/valsartan and vericiguat. In patients not on sacubitril/valsartan, the primary outcome occurred in 760 patients with vericiguat and 718 patients on placebo (HR 0.90, CI 0.81-0.99). In patients with concomitant use of vericiguat and secobarbital/valsartan, the primary outcome occurred in 134 patients in the vericiguat group and 152 patients in the placebo group (HR 0.88, nonsignificant CI 0.70-1.11).
The subgroup analysis revealed that vericiguat may benefit patients with moderately reduced estimated glomerular filtration rate (eGFR). In patients with an eGFR between 30 mL/min/1.73 m2 and 60 mL/min/1.73 m2, the primary outcome occurred in 392 patients in the vericiguat group and 455 patients in the placebo group (HR 0.84, CI 0.73-0.96). Patients with an eGFR <30 or >60 mL/min/1.73 m2 did not appear to benefit significantly from adding vericiguat.
Patients with reduced EF were more likely to benefit from adding vericiguat than those with a mildly reduced or preserved EF. For patients with an EF of less than 40%, the primary outcome occurred in 773 patients in the vericiguat group and 851 in the placebo group (HR 0.88, CI 0.80-0.97). For those with an EF 40% or greater, the primary outcome occurred in 119 patients in the vericiguat group and 117 in the placebo group (HR 1.05, CI 0.81-1.36).
CONCLUSION
The most recent HF guidelines recommend the use of vericiguat in patients treated with guideline-directed medical therapy with an EF still <35%. The results from the VICTORIA trial demonstrate that patients most likely to benefit from the addition of vericiguat are those aged younger than 75 years, who have moderately reduced kidney function, are NYHA class III or IV, or unable to tolerate an ARNI.
REFERENCES
1. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032.2. Swedberg K, Kjekshus J, CONSENSUS trial study group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435.3. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349-1355.4. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
5. Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.6. McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
7. Yancey CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation. 2017;136:e137-e161.8. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
9. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.10. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008.
11. Armstrong PW, Pieske B, Antrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883-1893.12. Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA. 2015;314(21):2251-2262.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






