US Pharm. 2022;47(11):36-71.
ABSTRACT: Rheumatoid arthritis (RA) is caused by an autoimmune response in which the immune system attacks the synovial tissues of the joints and other tissues. Therapy focuses on a treat-to-target approach, usually involving the initiation and titration of disease-modifying antirheumatic drugs (DMARDs) with a goal of disease remission or significant symptom alleviation, depending on the severity. Methotrexate is the recommended DMARD for initiation in most patients, although its use depends on individual patient characteristics and shared decision making. Adding other DMARDs, including biological agents, may be considered as treatment progresses. Given the complicated dosing, adverse-event profiles, and potentially high costs associated with RA pharmacotherapy, pharmacists can perform useful services for both patients and prescribers.
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation of synovial tissue in the joints that results in joint pain and stiffness. Initially, the small joints of the hands and wrists are most commonly affected, with potential progression to the knees, ankles, shoulders, elbows, and other joints. Over time, RA can have extraarticular manifestations involving other bodily systems and organs, such as the heart, lungs, and kidneys.1-3
Epidemiology
RA is one of the most common autoimmune diseases, with an estimated prevalence of about 0.5% in the United States (compared with 0.24% globally).4,5 RA generally develops in middle age; the rate of development prior to age 50 years is <1%, and the prevalence increases with increasing age.6 Women are more commonly affected, with an estimated lifetime risk of 3.6% compared with 1.7% for men.6 Genetics and family history also play a role in lifetime risk, as the incidence of RA is 3.02 times and 4.64 times greater in persons with affected parents and siblings, respectively, than in those without affected first-degree relatives.7
External factors have also been demonstrated to affect lifetime incidence of developing RA, with smoking being the best established of these. Studies have shown that cigarette smoke can almost double a person’s risk of developing RA.8 Obesity, diet, and occupational silica-dust exposure are other external factors associated with increased risk of RA.9
Pathophysiology
RA is most accurately described as an immune-mediated inflammatory disease.3 In RA, the immune system is unable to distinguish self tissue from nonself tissue, so it attacks the synovial tissues and other connective tissues, initiating an immune cascade and inflammatory-pathway dysregulation. Factors that trigger this inflammatory process are not well understood, but normal immune processes are negatively impacted. This can result in inflammation, cell proliferation, and destruction of tissue and fluids.1-3,10
Citrulline is an amino acid generated by posttranslational modification of arginyl residues by peptidyl arginine deiminases. The process of citrullination is required for skin formation and other physiological functions. In RA patients, an autoimmune response against citrullinated peptides occurs that is mediated by anticitrullinated peptide antibodies (ACPA).1-3,11
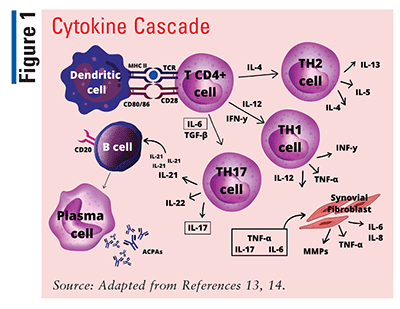
It is common for T-cell abnormalities to be present in patients with RA. One such abnormality is exaggerated cluster of differentiation (CD) 4+ T-cell activity, suggesting that abnormal T-cell activation may contribute to disease pathogenesis. Dendritic cells express cytokines, human leukocyte antigen class II molecules (major histocompatibility complex [MHC] II), and costimulatory molecules CD80/86; they are also involved in antigen presentation and T-cell activation.12
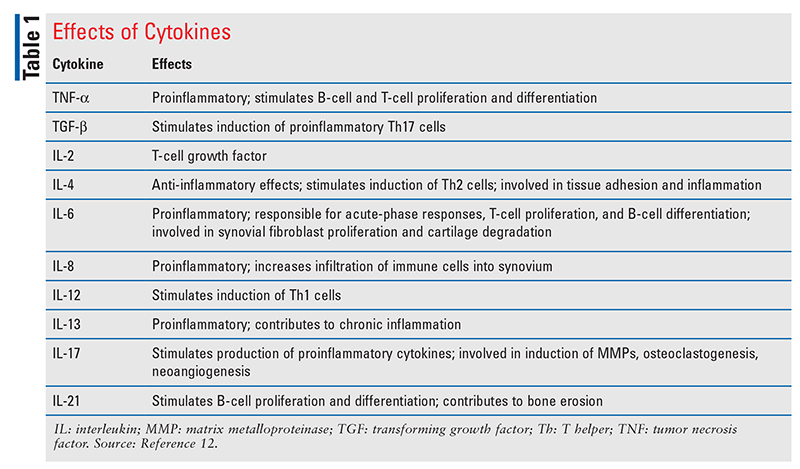
T cells require two signals for activation; the first signal is antigen-specific, involving the MHC, and the second signal involves the interaction of CD80/86 on the dendritic cell with CD28 on the T cell. Once the T cell is activated by the dendritic cell, the T cell secretes more inflammatory cytokines (interleukin [IL]-4, IL-6, IL-12), promoting cell proliferation (TABLE 1).12 When these T cell–derived cytokines are released, more T cells are activated, stimulating B-cell differentiation into plasma cells. This activation leads to a cascade of systemic effects, including reduced production of hyaluronan (a major synovial fluid lubricant); increased matrix metalloproteinase production, which degrades the matrix of articular cartilage and leads to bone resorption; and cartilage damage caused by plasma-cell antibody-mediated immune complexes (FIGURE 1).13,14 The resulting joint damage recruits more neutrophils, T cells, and B cells into the joint space, causing angiogenesis and hyperplasia of the synovial membrane.1-3,12,13,15,16
Diagnosis and Testing
The American College of Rheumatology (ACR)/European League Against Rheumatism 2010 classification criteria for RA are used to classify patients with definite swelling of at least one joint on clinical examination whose synovial inflammation is not better accounted for by another diagnosis.17 Domains A through D are assigned points based on joint involvement, symptom duration, serology results, and acute-phase reactant status. Points from each of the domains are added up to obtain the total score. A total score of 6 or greater is required to classify a patient with definite RA; however, not all patients with RA will score this high initially. The classification criteria use various laboratory findings to assign points for diagnosis, including rheumatoid factor (RF), ACPA, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR).17
There is no single laboratory test for RA, which makes definitive diagnosis challenging.1-3,17 RF, an antibody specific for immunoglobulins, is detectable in 60% to 90% of RA patients.18 The presence of RF is not necessarily diagnostic of RA, as it may occur in other connective-tissue diseases and chronic infections, and even in healthy persons. Compared with RF, ACPA have similar sensitivity but greater specificity. The fact that ACPA can be detected up to 15 years before the onset of clinical RA symptoms indicates a preclinical disease phase in which immunologic activation is already occurring.3 If both RF and ACPA are positive, the sensitivity and specificity of the diagnosis increase substantially.17,19 The presence of antibodies in RA is known as seropositive RA, which is an important classification for diagnosis. Inflammatory markers such as ESR and CRP tend to be elevated in RA but are nonspecific for diagnostic purposes.1,2,17
Treatment
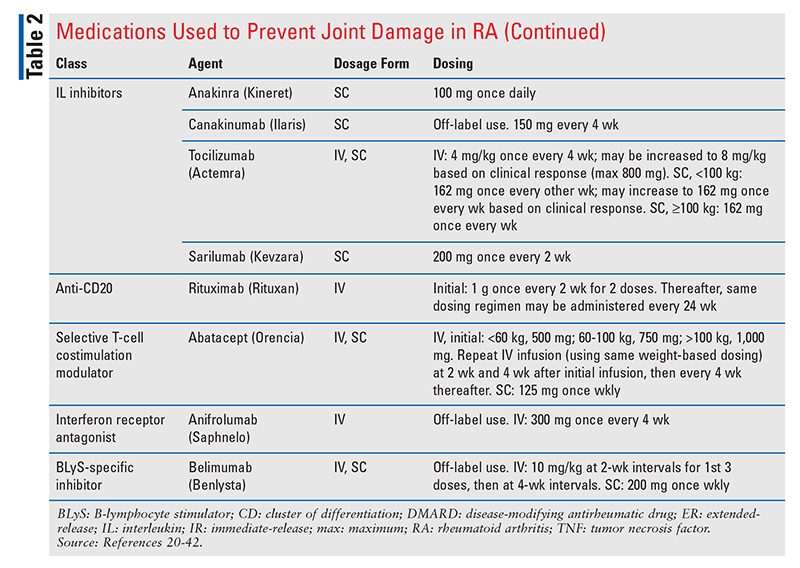
A broad range of therapy options are available for RA. The treatment goals are to reduce joint inflammation, prevent further joint destruction, and reduce associated pain. The medications used to prevent joint damage are categorized into synthetic and biological DMARDs (TABLE 2).20-42
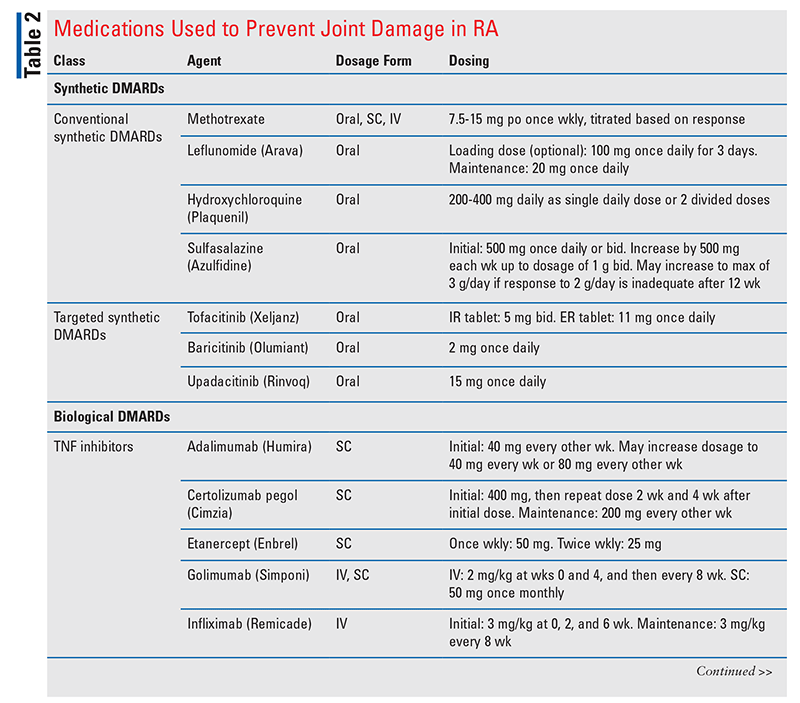
DMARD-Naïve Patients With Moderate-to-High Disease Activity: Methotrexate (MTX) monotherapy is the treatment of choice for treatment-naïve patients who have moderate-to-high disease activity.21 Based largely on well-established efficacy and safety data as well as the generally low cost of treatment, MTX is recommended over other conventional synthetic DMARDs (csDMARDs), targeted synthetic DMARDs (tsDMARDs), biological DMARDs (bDMARDs), and associated combination therapies. This is an expanded recommendation, as previous guidelines did not contain a recommendation for a preferred DMARD in newly diagnosed RA patients with moderate-to-high disease activity; the older guidelines recommended only that a single DMARD be initiated instead of double or triple therapy.21,43-45
Additionally, avoidance of routine concomitant glucocorticoid therapy is conditionally recommended over systemic use in treatment-naïve patients.22 The only situation in which the addition of glucocorticoids to csDMARD monotherapy should be considered is when the DMARD’s onset of action may not be suitable for timely treatment of the patient. Glucocorticoid therapy may serve as an appropriate short-term bridge until an adequate response can be established. This treatment should prioritize the lowest effective dose in order to prevent long-term adverse effects (AEs). To avoid such toxicities, glucocorticoid use should be limited to a duration of no more than 3 months.22
DMARD-Naïve Patients With Low Disease Activity: In the setting of low disease activity, MTX as DMARD monotherapy is no longer the preferred first-line treatment.21,46 Instead, based on its generally more favorable AE profile, hydroxychloroquine is now viewed as the best-supported treatment option in the absence of more severe disease. For similar reasons, sulfasalazine is also conditionally preferred over MTX in this setting. However, MTX may be considered in patients on the higher end of the low-disease-activity range or in patients who possess identified negative prognostic factors. Leflunomide is not recommended over other csDMARDs owing to the associated expense and the narrower therapeutic window versus MTX; therefore, it generally should be avoided.21,46,47
MTX Dosing and Administration: When MTX is initiated, its optimization should be prioritized before other DMARDs are added or agents are switched. However, even with this recommendation, the patient’s preference and experience should be considered in treatment selection.20,21
In general, the oral formulation of MTX is preferred for treatment of RA. Although the SC formulation may be more efficacious in some settings, the ease and convenience of oral administration are advantages considered to outweigh the possible benefits of SC administration.20,21 The dosage of oral MTX should be initiated at or titrated to at least 15 mg once weekly within 4 to 6 weeks. This is recommended over more conservative initiations and titrations reaching weekly doses of <15 mg. In situations of poor oral MTX tolerance, split oral dose regimens or weekly SC injections should be considered. These adjustments are preferable to switching to alternative DMARDs; however, it is recommended that patient preference be considered before making such changes.20,21
Modifying Treatment
Treat-to-Target: In RA patients who are taking DMARDs but are not achieving their therapy goals, the disease should be managed according to a treat-to-target approach. In these cases, a structured approach involving careful monitoring and adjustment should be used to optimize treatment and minimize disease activity. This is strongly recommended over usual care. In general, this approach emphasizes the dose optimization of individual DMARDs alongside the judicious addition of subsequent DMARDs when appropriate.48,49
In determining the approach to therapy, a shared decision-making process should be employed wherein the patient has autonomy in planning the treatment and management. The initiated treatment should be evaluated within 3 months to assess the tolerability and efficacy of the treatment plan. As the patient reaches the target goal, a tapering process should be considered once a 6-month duration is achieved. This practice, along with maintaining at least one DMARD at therapeutic dosing, may help minimize the risk of flares and possible severe damage associated with sudden discontinuation of DMARDs.48,49
Previous guidelines supported the use of triple therapy (a combination regimen of MTX, sulfasalazine, and hydroxychloroquine) in patients who failed MTX monotherapy. The 2021 ACR guideline recommends instead the addition of a bDMARD or tsDMARD to MTX monotherapy, citing the advantage of maximizing effective improvement in the shortest amount of time. This recommendation should be considered carefully in low-income patients or in patients with specific intolerances to certain DMARDs. In those already taking specific bDMARDs or tsDMARDs who are not at target, switching to a DMARD from another class is recommended over options within the same class.21,50
DMARD Discontinuation: Sudden cessation of DMARDs can lead to negative outcomes. In the short term, there is an increased risk of flares and acute pain and exacerbation; in the longer term, irreversible joint damage can occur. Therefore, abrupt discontinuation of DMARDs is generally not recommended. When it is desirable to discontinue a DMARD, this should be done in a tapering, stepwise manner under prescriber supervision. Before tapering is considered, at least one DMARD should remain therapeutic and the patient should be established at target disease activity for a minimum of 6 months.21
In general, dose reductions of stable DMARD therapy are not recommended. This is because of the risk of inciting flares. Assessment of the appropriateness of discontinuation should be made on an individual basis. If treatment must be reduced or discontinued, abrupt discontinuation should be avoided in order to prevent these AEs.21
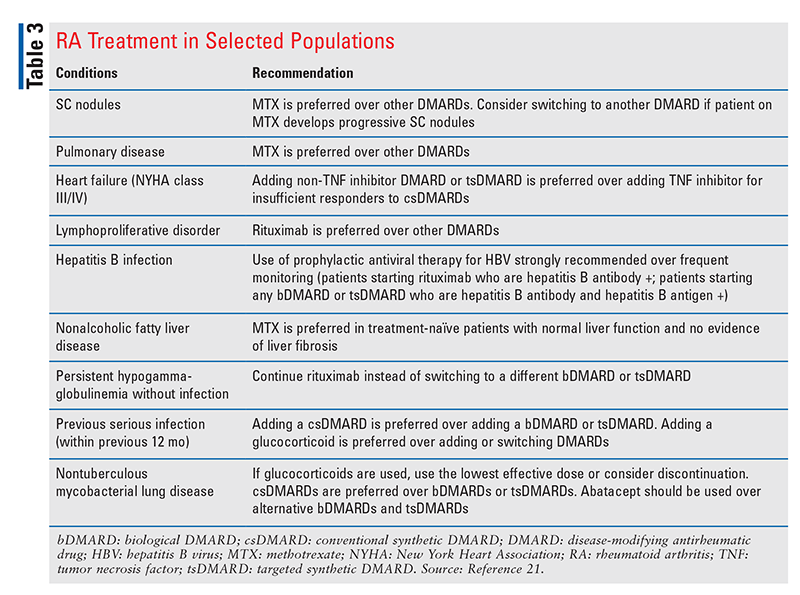
For patients on established triple therapy, gradual discontinuation of sulfasalazine is recommended over gradual discontinuation of hydroxychloroquine. This is because of the poorer treatment persistence with sulfasalazine based on its AE profile.21 If a patient on established MTX plus a bDMARD or tsDMARD wants to stop taking a DMARD, it is recommended to gradually discontinue the MTX instead of the bDMARD or tsDMARD, even though MTX is recommended monotherapy. This is because patients who are taking a combination of MTX and a bDMARD or tsDMARD likely had an inadequate response to MTX monotherapy and are at risk for undertreatment if tapered to MTX alone.21 See TABLE 3 for recommendations on the treatment of RA in selected populations.21
The Pharmacist’s Role
The management of RA can be complex. Given the complicated dosing, AE profiles, and potentially high costs associated with RA pharmacotherapy, pharmacists can perform useful services for both patients and prescribers. Pharmacist counseling can provide patients with essential information, including awareness of possible AEs, knowledge about drug interactions, and the importance of compliance with treatment. Additionally, pharmacists can offer prescribers valuable perspectives in that their knowledge and experience can inform drug selection based on specific patient needs, such as intolerances, interactions, and unnecessary drug costs.51
REFERENCES
1. Chauhan K, Jandu JS, Goyal A, Al-Dhahir MA. Rheumatoid arthritis. In StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022 Jan-.
2. Aletaha D, Smolen J. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360-1372.
3. Johns Hopkins Arthritis Center. RA pathophysiology. www.hopkinsarthritis.org/arthritis-info/rheumatoid-arthritis/ra-pathophysiology-2/. Accessed August 29, 2022.
4. Hunter TM, Boytsov NN, Zhang X, et al. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551-1557.
5. Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1316-1322.
6. Crowson CS, Matteson EL, Myasoedova E, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63(3):633-639.
7. Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60(3):661-668.
8. Ishikawa Y, Terao C. The impact of cigarette smoking on risk of rheumatoid arthritis: a narrative review. Cells. 2020;9(2):475.
9. Myasoedova E, Davis J, Matteson EL, Crowson CS. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann Rheum Dis. 2020;79(4):440-444.
10. Kuek A, Hazleman BL, Ostör AJK. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83(978):251-260.
11. Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, et al. Citrullination and autoimmunity. Autoimmun Rev. 2015;14(6):490-497.
12. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor b, and TNF-a: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984-1010.
13. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205-2219.
14. Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18(suppl 13):S295-S302.
15. Zhang J, Hu X, Dong X, et al. Regulation of T cell activities in rheumatoid arthritis by the novel fusion protein IgD-Fc-Ig. Front Immunol. 2020;11:755.
16. Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3(12):973-983.
17. Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford). 2012;51(suppl 6):vi5-vi9.
18. Tiwari V, Jandu JS, Bergman MJ. Rheumatoid factor. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022 Jan-.
19. Pope JE, Movahedi M, Rampakakis E, et al. ACPA and RF as predictors of sustained clinical remission in patients with rheumatoid arthritis: data from the Ontario Best practices Research Initiative (OBRI). RMD Open. 2018;4(2):e000738.
20. Bello AE, Perkins EL, Jay R, Efthimiou P. Recommendations for optimizing methotrexate treatment for patients with rheumatoid arthritis. Open Access Rheumatol. 2017;9:67-79.
21. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73(7):924-939.
22. Kumar P, Banik S. Pharmacotherapy options in rheumatoid arthritis. Clin Med Insights Arthritis Musculoskel Disord. 2013;6:35-43.
23. Maddison P, Kiely P, Kirkham B, et al. Leflunomide in rheumatoid arthritis: recommendations through a process of consensus. Rheumatology (Oxford). 2005;44(3):280-286.
24. Plaquenil (hydroxychloroquine) package insert. St. Michael, Barbados: Concordia Pharmaceuticals Inc; January 2017.
25. Azulfidine (sulfasalazine) package insert. New York, NY: Pfizer Inc; August 2021.
26. Xeljanz (tofacitinib) package insert. New York, NY: Pfizer Inc; May 2018.
27. Olumiant (baricitinib) package insert. Indianapolis, IN: Lilly USA, LLC; May 2022.
28. Rinvoq (upadacitinb) package insert. North Chicago, IL: AbbVie Inc; January 2022.
29. Humira (adalimumab) package insert. North Chicago, IL: Abbott Laboratories; December 2011.
30. Cimzia (certolizumab pegol) package insert. Smyrna, GA: UCB, Inc; January 2017.
31. Emery P, Breedveld F, van der Heijde D, et al. Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomized study. Arthritis Rheum. 2010;62(3):674-682.
32. Genovese MC, Bathon JM, Martin RW, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46(6):1443-1450.
33. Simponi (golimumab) package insert. Horsham, PA: Janssen Biotech, Inc; December 2011.
34. Remicade (infliximab) package insert. Horsham, PA: Janssen Biotech, Inc; November 2013.
35. Kineret (anakinra) package insert. Stockholm, Sweden: Swedish Orphan Biovitrum AB; December 2012.
36. Alten R, Gomez-Reino J, Durez P, et al. Efficacy and safety of the human anti-IL-1b monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, phase II, dose-finding study. BMC Musculoskel Disord. 2011;12:153.
37. Actemra (tocilizumab) package insert. South San Francisco, CA: Genentech, Inc; October 2013.
38. Kevzara (sarilumab) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; April 2018.
39. Rituxan (rituximab) package insert. South San Francisco, CA: Genentech, Inc; February 2010.
40. Orencia (abatacept) package insert. Princeton, NJ: Bristol-Myers Squibb; December 2013.
41. Saphnelo (anifrolumab-fnia) dosing and administration. www.saphnelo.com/hcp/dosing.html. Accessed August 29, 2022.
42. Benlysta (belimumab) package insert. Research Triangle Park, NC: GlaxoSmithKline; June 2018.
43. Curtis JR, Palmer JL, Reed GW, et al. Real-world outcomes associated with methotrexate, sulfasalazine, and hydroxychloroquine triple therapy versus tumor necrosis factor inhibitor/methotrexate combination therapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73(8):1114-1124.
44. Erhardt DP, Cannon GW, Teng CC, et al. Low persistence rates in patients with rheumatoid arthritis treated with triple therapy and adverse drug events associated with sulfasalazine. Arthritis Care Res (Hoboken). 2019;71(10):1326-1335.
45. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1-26.
46. Singh JA, Saag KJ, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1-25.
47. Ishaq M, Muhammad JS, Hameed K, Mirza AI. Leflunomide or methotrexate? Comparison of clinical efficacy and safety in low socio-economic rheumatoid arthritis patients. Mod Rheumatol. 2011;21(4):375-380.
48. Solomon DH, Bitton A, Katz JN, et al. Treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol. 2014;66(4):775-782.
49. Smolen JS, Aletaha D, Bijlsma JWJ, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631-637.
50. Sauer BC, Teng CC, Tang D, et al. Persistence with conventional triple therapy versus a tumor necrosis factor inhibitor and methotrexate in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2017;69(3):313-322.
51. Bornstein C, Craig M, Tin D. Practice guidelines for pharmacists: the pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. Can Pharm J (Ott). 2014;147(2):97-109.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






