US Pharm.
2008;33(7):26-34.
Allergic rhinitis (AR) is a
common, often chronic, health condition associated with significant morbidity.1
Approximately 20% of adults and 40% of children have symptoms consistent with
the disease, which can range in severity from mild to moderate-severe.2
For its sufferers, AR can have a consequential impact on quality of life,
direct medical costs, and productivity. This article aims to provide an
overview of AR, its management principles, and the role of the pharmacist in
providing care for patients with AR.
Rhinitis is an inflammation of
the nasal mucosa.1 When associated with allergens, rhinitis
encompasses an even broader spectrum of signs and symptoms, including
rhinorrhea, nasal congestion, pruritus, sneezing, postnasal drip, and
sometimes nasal pain. Other upper respiratory organs, including the eyes,
ears, sinuses, and oropharynx may be involved, as characterized by symptoms of
ocular pruritus and watering, ear pain, recurrent sinusitis, and chronic
cough. Patients may also display characteristic physical exam findings such as
a darkening below the eyes ("allergic shiners") or a nasal crease caused by
constant rubbing ("allergic salute").1,3-7 The symptoms
of AR may be difficult to distinguish from nonallergic forms; however, more
prominent nasal pruritus and conjunctival irritation, as seen with AR, may
help differentiate. Additionally, when skin-prick testing is performed, the
presence of an immunoglobulin E (IgE)-mediated allergic reaction would lend
itself to a diagnosis of AR in the presence of symptoms and may aid in
allergen avoidance as a nonpharmacologic management strategy.6
Other types of rhinitis, such as hormonal and infectious, should also be ruled
out. The use of a patient diary to record rhinitis symptoms, their triggers,
and when they occur may help with diagnosis.
Depending on the temporal
relationship of allergy symptoms, AR may be further classified into two types. Seasonal
AR, sometimes called hay fever, manifests at a particular time of
the year, usually when allergens such as fungi and tree, grass, and weed
pollens are at the highest levels. Perennial AR is associated with
year-round symptoms and is typically related to indoor allergen exposure to
dust mites, animal proteins, cockroaches, and molds.1,5,6 AR may
also be classified by symptom frequency, either as intermittent
(symptoms lasting <4 days/wk for <4 wk) or persistent (symptoms
lasting >=4 days/wk for >=4 wk regardless of symptoms).8
Describing rhinitis symptoms
according to mild or moderate-severe disease may also be helpful for
treatment. Moderate-severe disease is associated with a minimum of one of the
following: sleep disturbance; impairment of school or work performance;
impairment of daily leisure or sports activities; or troublesome symptoms,
which may have a significant emotional impact due to their severity.9
Moderate-severe AR generally requires more intensive medical management.
Although more children and
adolescents suffer from symptoms of AR, symptoms tend to have a bimodal peak
during childhood and middle age, but can begin at any time.10 In
addition to allergen exposure, several risk factors predispose a person to
developing AR (Table 1). AR commonly occurs in the presence of other
allergic-type disorders as well, including sinusitis, asthma, eczema, and
allergic conjunctivitis, and can exacerbate their severity. AR can also cause
otitis media as a result of eustachian tube dysfunction and may result in
migraine headaches or anosmia if improperly managed.10

Management Principles
The goal of AR
treatment is to reduce or eliminate inflammation-associated allergy symptoms.
Additionally, improving quality of life and productivity are equally
important, as is minimizing medication-related adverse effects.
AR can be treated by employing
both nonpharmacologic and pharmacologic modalities for symptom management, as
well as immunotherapy. Nonpharmacologic therapy should be implemented in all
patients with AR to help decrease symptom severity; however, most patients
will not receive complete resolution of symptoms with nondrug therapies alone.
Allergen avoidance is
recommended as one of the main nonpharmacologic strategies for managing
symptoms of AR, although for most patients it is impractical. Reducing
environmental exposure to allergens by keeping windows closed and minimizing
outdoor activities during the pollen season may help reduce symptoms.
Minimizing mold growth through proper cleaning and dehumidification can help
patients with a mold allergy. Other indoor allergens, such as house dust
mites, animal dander, and insects should be minimized or avoided through
proper cleaning measures, selecting allergen-proof covers and construction
materials, and by utilizing high-efficiency particulate air (HEPA) filters.6
Pharmacologic therapy and
immunotherapy can help patients achieve symptom resolution to improve their
quality of life. Patients who experience seasonal AR may be able to anticipate
the onset of symptoms and use pharmacologic therapy immediately prior to and
during allergy season.
Medication Classes
Medication classes
used to treat AR include intranasal corticosteroids, oral antihistamines,
topical antihistamines, leukotriene modifiers, topical mast cell stabilizers,
topical anticholinergics, and decongestants. The following discussion and Table
2 provide an overview of these drugs.
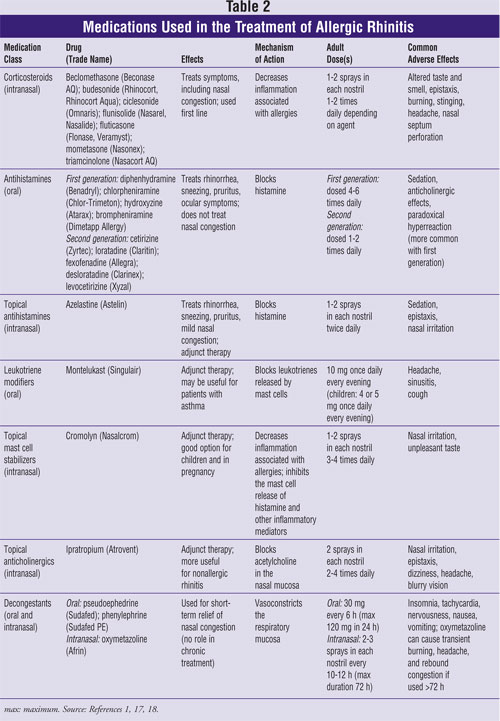
Intranasal Corticosteroids:
Intranasal corticosteroids are first line for the treatment of AR.1,2,4,11-15
This class of medications is the most effective compared to other drugs for
controlling the symptoms associated with AR, including nasal congestion, nasal
discharge, pruritus, sneezing, and postnasal drip.11-12,16
Available by prescription only, specific agents include beclomethasone
(Beconase AQ), ciclesonide (Omnaris), budesonide (Rhinocort, Rhinocort Aqua),
mometasone (Nasonex), triamcinolone (Nasacort AQ), and fluticasone furoate
(Veramyst), as well as flunisolide (Nasarel, Nasalide) and fluticasone
propionate (Flonase), both of which are now available as generics. Veramyst,
the newest FDA-approved intranasal corticosteroid for the treatment of AR,
treats both nasal and ocular symptoms.17 In regard to efficacy, no
significant difference exists between agents; however, steroids do differ in
potency. When comparing intranasal corticosteroids to oral antihistamines,
intranasal corticosteroids provide better overall relief of symptoms, which is
supported by numerous studies and meta-analyses.2,12
Intranasal corticosteroids
work by decreasing inflammation associated with AR. Ideally, they should be
started before the onset of seasonal symptoms and used every day.1
Most of the intranasal corticosteroids are dosed one to two sprays in each
nostril once or twice daily. If the medication is dosed once daily, it should
be administered at night, due to the fact that nasal inflammation is greater
during the night than during the day. Optimally, therapy should be started at
the maximum dose for the patient's age, then stepped down at one-week
intervals to the lowest effective dose.12 Some patients may even
experience relief of symptoms with administration every other day. This
concept is especially useful for children, who may benefit from use of
intranasal corticosteroids as needed.12
The most common adverse
effects of intranasal corticosteroids include transient burning after
application, epistaxis, nasal pruritus, headache, and pharyngitis.1,4,12
Systemic adverse effects, including insomnia, nervousness, increased appetite,
indigestion, headache, hyperglycemia, and diaphoresis, can occur, especially
if the steroid is used at high doses for an extended period of time.12,18
The intranasal steroids are
divided into three generations depending on their bioavailability.
Beclomethasone and flunisolide, two first-generation intranasal
corticosteroids, are more bioavailable and tend to produce more systemic
adverse effects than other intranasal corticosteroids.12 The other
steroids fall into second- and third-generation categories and tend to have
limited systemic adverse effects. Hypothalamic-pituitary-adrenal (HPA) axis
suppression and effects on growth in children are potential concerns with
topical corticosteroids. These effects have been evaluated in various studies,
and the second- and third-generation intranasal steroids, at recommended
doses, show no or limited effects on HPA axis suppression and decreased
vertical growth of children. Furthermore, agents that are dosed once daily are
preferred in children in order to minimize the amount of steroid the child
receives.12
Oral Antihistamines:
Oral antihistamines are
effective at reducing most, but not all, symptoms of AR.4,11 This
class of medications specifically treats pruritus, sneezing, rhinorrhea, and
watery eyes.4,11,13,15 The drugs do not combat the nasal congestion
associated with AR.1,4,11-13
Oral antihistamines are
divided into two generations, which differ by their adverse-effect profile and
dosing. All of the agents are equally efficacious when compared to each other.12
The first-generation oral antihistamines include diphenhydramine
(Benadryl), chlorpheniramine (Chlor-Trimeton), hydroxyzine (Atarax), and
bromphenir amine (Dimetapp Allergy). These agents are dosed every four to six
hours and cause significant sedation, their limiting adverse effect.12,18
Anticholinergic adverse effects, most commonly dry mouth, dry eyes, urinary
retention, and confusion, can occur when taking a first-generation oral
antihistamine.12 Confusion is prevalent when used in the elderly
and can be detrimental to their overall well-being. When children take these
medications, they can experience a paradoxical stimulation reaction;
therefore, it is not recommended that these agents be used in children less
than 2 years of age.18
Second-generation
oral antihistamines have all of the benefits of first-generation agents
without the sedation and anticholinergic adverse effects. Second-generation
agents are generally dosed once or twice daily and do not cross the blood
brain barrier; therefore, they do not cause sedation to the extent of the
first-generation agents. Second-generation antihistamines are well tolerated
and, before generic and OTC versions were introduced, were significantly more
expensive than first-generation medications. Agents include cetirizine
(Zyrtec) and loratadine (Claritin), available OTC, and fexofenadine (Allegra),
desloratadine (Clarinex), and levocetirizine (Xyzal), available by
prescription only. Some of these medications are also found in combination
with pseudoephedrine to treat nasal congestion (e.g., Allegra-D, Claritin-D,
Zyrtec-D).14 Patients with mild-to-moderate symptoms may achieve
adequate relief by using only oral antithistamines.12
Topical Antihistamines:
Azelastine (Astelin), an
intranasal antihistamine, has the same properties of oral antihistamines. It
does not treat nasal congestion equally to intranasal corticosteroids or
decongestants; however, it may provide some relief of nasal congestion.12
Azelastine is dosed twice daily and can be used in children 5 years of age and
older.18 Common adverse effects include headache, sedation, and
bitter taste after administration. This medication does not offer any benefits
over oral antihistamines and intranasal corticosteroids.1,18
Leukotriene Modifiers:
Antileukotriene agents
are available to treat allergies and asthma. Montelukast (Singulair) is the
only agent in this class approved for treatment of AR. A review of randomized
clinical trials has shown that montelukast is comparable to loratadine in
relieving symptoms associated with AR and may be equally efficacious to a
combination of an intranasal corticosteroid with a second-generation oral
antihistamine.12,15 Montelukast can be used in children as young as
2 years for AR and may be most beneficial in patients who also have asthma.12,18
Furthermore, patients who do not tolerate intranasal corticosteroids may
receive relief of symptoms from either montelukast alone or in combination
with an oral antihistamine.12
Topical Mast Cell
Stabilizers: Cromolyn
(Nasalcrom) nasal spray is a mast cell stabilizer available OTC that is
effective in decreasing symptoms of AR if used regularly.15 Mast
cell stabilizers decrease inflammation associated with allergies by inhibiting
the mast cell release of histamine and other inflammatory mediators.1,12
This medication can be dosed 30 minutes prior to exposure; therefore, it may
be particularly useful for patients with a specific known allergy who plan on
coming in contact with that allergen. More efficacious than placebo, cromolyn
is less effective when compared to intranasal corticosteroids and oral
antihistamines.12,15 One drawback to its use is that cromolyn must
be dosed four times daily to achieve symptom relief.1,12 Nasal
pruritus, irritation, sneezing, and epistaxis are the most common adverse
effects associated with use of cromolyn.1,4 Overall, the medication
is well tolerated, which makes it especially useful for treating children with
AR.12
Topical Anticholinergics:
Topical anticholinergics,
specifically ipratropium (Atrovent) nasal spray, may be effective in the
treatment of rhinorrhea associated with AR.13 It generally does not
relieve nasal pruritus or nasal congestion and therefore may be most
beneficial in the treatment of nonallergic rhinitis or postnasal drip.1,15
Common adverse effects associated with its use include headache and epistaxis.4
To achieve maximum effectiveness, ipra tropium should be dosed two sprays in
each nostril two to four times daily and initiated before symptom onset.4,11
Decongestants:
Decongestants,
medications marketed specifically to relieve nasal congestion, are available
in both oral and topical dosage forms.11 Oral decongestants include
pseudoephedrine (e.g., Sudafed), available behind the counter or by
prescription, and phenylephrine (e.g., Sudafed PE), available OTC. Common
adverse effects associated with the use of decongestants include insomnia,
anorexia, tachycardia, and nervousness.11
The most common topical OTC
decongestant is oxymetazoline (Afrin) nasal spray. Nasal decongestants are not
recommended for the long-term treatment of AR and can only be used for a short
period of time before rebound congestion occurs.12 Patients are at
risk for developing rebound congestion, also known as rhinitis medicamentosa,
if topical decongestants are used incorrectly. The maximum duration of
continued use of a topical decongestant is less than 72 hours (3 days).
Patients using topical decongestants should understand that if the agents are
used longer than three days, rebound congestion will develop. Furthermore, if
an intranasal corticosteroid is started, the topical decongestant should be
discontinued.11 If extreme nasal congestion occurs, relief may be
found with short-term use of nasal decongestants. Overall, decongestants have
no role in the chronic treatment of AR.
Immunotherapy
Immunotherapy, or
hyposensitization, may be used in the treatment of moderate-severe AR in those
who do not receive adequate relief with combination therapy including an
intranasal corticosteroid, and in patients who have coexisting diseases such
as asthma or sinusitis.1,4,13,15 Immunotherapy is directed toward a
specific known allergen and involves weekly subcutaneous injections of
increasing doses of an allergen until a maintenance dose is found, after which
it is administered at two- to six-week intervals.1,4,13,15 Although
this disease-modifying treatment takes several years to complete, patients may
experience relief for years following therapy.1,16
Immunotherapy can be quite
dangerous, as the risk of an anaphylactic reaction is high.16
Systemic reactions occur in approximately 5% to 10% of patients receiving
immuno therapy.4 However, the efficacy of immunotherapy for
AR is well documented; patients receiving this treatment will experience
improvement in their symptoms.1
A new approach to
immunotherapy is to administer the allergen sublingually.4 This
method is currently being used in Europe with no reports of systemic reactions
recorded to date.4 Sublingual immunotherapy is not available in the
United States. Immunotherapy is contraindicated in patients with severe
cardiovascular disease and severe asthma.13 Agents discussed
earlier can be used in combination with immunotherapy for complete relief of
symptoms.
Special Populations
Children:
Children may experience symptoms of AR due to a variety of other conditions.
To be sure that AR is the correct diagnosis, children should be evaluated by a
health care provider before initiating any pharmacologic therapy, including
OTC medications. Overall, oral antihistamines and cromolyn are first-line
agents for the treatment of AR in children. When choosing an oral
antihistamine, comparing the adverse-effect profiles of the generations will
lead one to select a second-generation oral antihistamine. As discussed
earlier, second-generation antihistamines are associated with less drowsiness
and can be dosed once to twice daily for most individuals.11
Cetirizine has recently gained OTC status and is approved for use in children
aged 6 months and older, although its labeling states that drowsiness may
occur.19 Cromolyn is considered first line in children and is
approved for use in children 2 years and older.13 Cromolyn may have
to be used for two to four weeks before maximum benefit is seen.18
As with the treatment of AR in
adults, intranasal corticosteroids are an excellent treatment option for
children with severe symptoms. Intranasal corticosteroids control sneezing,
rhinorrhea, nasal pruritus, and congestion; however, with the exception of
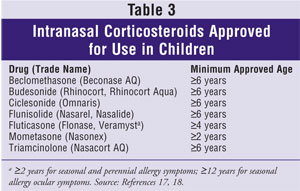
Veramyst, they have no effect on ocular symptoms.11,17 The
intranasal corticosteroids approved for use in children are listed in Table
3.
Controversy continues about
the use of corticosteroids in children. Current evidence suggests that the use
of long-term intranasal corticosteroids in children has an excellent
risk-benefit profile. The FDA states that the benefit of using intranasal
corticosteroids in children for AR clearly outweighs the risk of children not
attaining their perceived height. Children should be continually monitored
while using intranasal corticosteroids, and other therapeutic options should
also be implemented in order to use the lowest effective dose of steroid as
possible.11
Parents should be cautioned on
the use of OTC products for symptom relief in children. The FDA recently
released a warning regarding the use of cold, cough, flu, and allergy products
in this population, and currently does not recommend the use of these products
in children and infants under the age of 2.20 The appropriateness
of these agents in children aged 2 to 11 years is under investigation.
Inappropriate use has resulted in potentially dangerous and life-threatening
adverse effects. Specific products that were marketed toward this age group
have been voluntarily withdrawn from the market. Parents who use these
products in their children should only do so as specifically directed by a
health care provider, and under close supervision.
Pregnancy:
Treating chronic illness during pregnancy always presents a challenge.
Choosing not to treat symptoms of AR during pregnancy will not harm the woman
or her developing fetus. However, treatment can improve a pregnant woman's
quality of life, and the decision to treat should be left up to the woman and
her health care provider. Symptoms of AR often worsen during pregnancy partly
due to increased blood flow to nasal turbinates; however, some women may
experience resolution of their chronic symptoms of AR during pregnancy.15
As with all patients with AR, nonpharmacologic therapy should be implemented
first, and if symptoms do not resolve, pharmacologic therapy can then be added.
Cromolyn, an intranasal mast
cell stabilizer, is first line for the treatment of AR during pregnancy.16
This medication has been given a Pregnancy Risk Category B, which means that
no fetal harm has been demonstrated in animal or human studies.18
Oral antihistamines can also be used during pregnancy. Chlorpheniramine, a
first-generation antihistamine, has the most safety data supporting its use
during pregnancy. This medication is dosed every four hours to provide 24-hour
relief. Second-generation oral antihistamines that are Category B are
loratadine, cetirizine, and levocetirizine. These antihistamines are generally
chosen ahead of chlorpheniramine because they are less sedating and dosed once
daily.
Intranasal corticosteroids can
also be used during pregnancy if the above options are not providing enough
relief. Most of the steroids are Category C, which means that no studies have
been performed in humans or animals or that studies in animals have
demonstrated fetal harm. The exception is budesonide nasal spray, which
is Category B.18 Controversy remains about using intranasal
corticosteroids during pregnancy. Even though these medications have been
proven safe during pregnancy, recommendations still point women to using
possibly less-effective medications that have a better-established history in
terms of safety during pregnancy.16

The Pharmacist's Role
Pharmacists provide
care to patients suffering from AR and are in a position to help patients
maximize the use of medications, minimize adverse effects, and improve quality
of life. Nonpharmacologic modalities should be recommended to all patients
with AR to lessen symptoms. Pharmacists should use their expertise in
selecting a product to treat symptoms of AR and to counsel on the proper
technique of using a nasal spray (Table 4). Furthermore, pharmacists
can recommend therapies for children and pregnant women so they are treated
successfully and safely. AR is a common condition that impacts its sufferers
greatly. Pharmacists can offer medication options and nondrug therapies to
help manage the disease.
REFERENCES
1. Divya S, Secord
E, Deepak K. Allergic rhinitis. Clin Pediatr. 2007;46:401-407.
2. Allergy statistics.
American Academy of Allergy, Asthma & Immunology.
www.aaaai.org/media/resources/media_kit/allergy_statistics.stm. Accessed March
11, 2008.
3. Management of
Allergic and Nonallergic Rhinitis. Summary. Evidence Report/Technology
Assessment: Number 54. May 2002. Rockville, MD: Agency for Healthcare Research
and Quality. www.ahrq.gov/clinic/epcsums/rhinsum.htm. Accessed June 10, 2008.
4. Plaut M, Valentine
MD. Clinical practice. Allergic rhinitis. N Engl J Med.
2005;353:1934-1944.
5. Prenner BM, Schenkel
E. Allergic rhinitis: treatment based on patient profiles. Am J Med.
2006;119:230-237.
6. Rosenwasser LJ.
Treatment of allergic rhinitis. Am J Med. 2002;113(suppl 9A):17S-24S.
7. Spector SL, Nicklas
RA, Chapman JA, et al. Symptom severity assessment of allergic rhinitis: part
1. Ann Allergy Asthma Immunol. 2003;91:105-114.
8. Allergic and
nonallergic rhinitis. Chapter 76. In: Adkinson NF, Yunginger JW, Busse WW, et
al, eds. Middleton's Allergy: Principles and Practice. 6th ed.
St. Louis, MO: Elsevier; 2008. www.mdconsult.com. Accessed March 12, 2008.
9. Bousquet J, Van
Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J
Allergy Clin Immunol. 2001;108:S147-S334.
10. DeShazo RD, Kemp
SF. Clinical manifestations and evaluation of allergic rhinitis and
rhinosinusitis. UpToDate. www.uptodateonline.com.proxy.uwlib.uwyo.edu.
Accessed March 7, 2008.
11. Dykewicz MS,
Fineman S. Executive summary of Joint Task Force Practice Parameters on
Diagnosis and Management of Rhinitis. Ann Allergy Asthma Immunol.
1998;81:463-468.
12. DeShazo RD, Kemp
SF. Management of allergic rhinitis. UpToDate.
www.uptodateonline.com.proxy.uwlib.uwyo.edu. Accessed March 7, 2008.
13. Allergic rhinitis.
Allergies, anaphylaxis, and systemic mastocytosis. Chapter 298. In: Fauci AS,
Braunwald E, Kasper DL, et al, eds. Harrison's Principles of Internal
Medicine. 17th ed. New York, NY: McGraw-Hill; 2005.
www.accesspharmacy.com. Accessed March 11, 2008.
14. The Allergy Report.
American Academy of Allergy, Asthma & Immunology. www.aaaai.org. Accessed
March 12, 2008.
15. Chronic illnesses
in pregnancy. Gynecologic and obstetric disorders. Chapter 76. In: Dipiro JT,
Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach.
6th ed. New York, NY: McGraw-Hill; 2005. www.accesspharmacy.com. Accessed
March 11, 2008.
16. Courtney AU. Treatment
of Allergic Rhinitis in Adults. American Academy of Family Physicians.
Home study: a self-assessment program. Clinical update 346. March 2008.
17. DRUGDEX
Evaluations. Thomson Micromedex. Greenwood Village, CO: Thomson Healthcare;
2008. www.thomsonhc.com. Accessed March 11, 2008.
18. Lacy CF, Armstrong
LL, Goldman MP, Lance LL, eds. Lexi-Comp's Drug Information Handbook.
15th ed. Hudson, OH: Lexi-Comp, Inc; 2007.
19. Zyrtec (cetirizine)
package insert. Fort Washington, PA: McNeil-PPC, Inc; 2007.
20. FDA recommends that
over-the-counter (OTC) cough and cold products not be used for infants and
children under 2 years of age. Public health advisory: nonprescription cough
and cold medicine use in children. Food and Drug Administration. January 17,
2008. www.fda.gov/cder/drug/advisory/cough_cold_2008.htm. Accessed June 16,
2008.
To comment on this article, contact rdavidson@jobson.com.






