US Pharm. 2021;46(6):18-24.
ABSTRACT: Osteoporosis is characterized by aberrant bone remodeling that results in reduced bone mineral density, excessive bone loss, and increased fracture risk. Although osteoporosis most commonly affects postmenopausal women, it also occurs in older men. Some of the risk factors associated with male osteoporosis are age 70 years or older, reduced testosterone, sedentary lifestyle, poor nutrition, and medications that impair healthy bone turnover. Osteoporosis is undertreated in men, and screening is not routine. Given that men experience decreased quality of life and have an increased mortality risk following a traumatic fracture event, it is imperative for pharmacists to be aware of risk factors, causes, and pharmacologic and nonpharmacologic management of male osteoporosis in order to counsel this patient segment.
Osteoporosis is characterized by dysregulated bone turnover wherein the rate of osteoclast activity (bone loss) outpaces that of osteoblast activity (bone production).1 Osteoporotic bone is weak and brittle, which increases the risk of fracture and related complications (e.g., limited mobility, depression, pain).2 Although osteoporosis occurs most frequently in postmenopausal women, it also affects approximately 2 million men in the United States.3
Male Osteoporosis
Several factors related to male osteoporosis have been established. Osteopenia (low bone density) typically manifests in older age, with age-related hypogonadism (in men, testosterone decline) being a primary reason for this.4 Additionally, osteoporosis often occurs secondary to comorbidities that impair healthy bone turnover.5 Vitamin and mineral deficiencies related to decreased dietary intake or malabsorption, as well as comorbidities that lead to increased mobilization of calcium from bone or reduced mineralization of bone, also play a role.5 For instance, vitamin D deficiency and consequent calcium deficiency and secondary hyperparathyroidism can result in substantial bone loss.6 Of particular relevance to the pharmacist is the fact that male osteoporosis can be induced by a wide array of medications, including aluminum-containing antacids, antiseizure drugs, chemotherapeutic agents, immunosuppressants, androgen-deprivation therapy (ADT), heparin, proton pump inhibitors (PPIs), selective serotonin reuptake inhibitors (SSRIs), corticosteroids, and antidiabetic drugs (thiazolidinediones [TZDs]); an excess of thyroid hormone (TH) also can cause it.7-18 It should also be kept in mind that men with a family history of osteoporosis are predisposed to developing this condition.19
Bone health is primarily ascertained by a bone mineral density (BMD) test. Men, however, are less likely than women to undergo routine screening.20 They are also less likely to receive treatment following a low-trauma fracture, which increases their risk of sustaining subsequent fractures, and low treatment rates persist even after a high-trauma fracture involving the hip.20 The importance of heightened vigilance in identifying risk factors and clinically diagnosing osteoporosis in men is underscored by morbidity and mortality data showing that although comparatively fewer men develop the disorder, they are more likely to die within a year of fracturing a hip.21
The pharmacist can work to improve patient outcomes by fostering increased awareness of osteoporosis prevalence in older men; advocating for bone-health screening in individuals who are at increased risk; providing useful information on effective measures for maintaining healthy bone; and facilitating the management of osteoporosis upon diagnosis. In addition to a discussion of standard pharmacotherapy, the pharmacist should counsel on the benefits of lifestyle modifications, such as consuming a diet rich in calcium and vitamin D, performing resistance exercises (weight-bearing or strength-training), refraining from smoking and alcohol consumption, and practicing fall-prevention strategies, for reducing risk and counteracting bone loss.22
Risk Factors and Causes
Because men experience worse outcomes following major fracture events and their bone health is not routinely screened, identification of risk factors and recognition of primary and secondary causes of osteoporosis are especially important. TABLE 1 summarizes risk factors associated with male osteoporosis.23,24
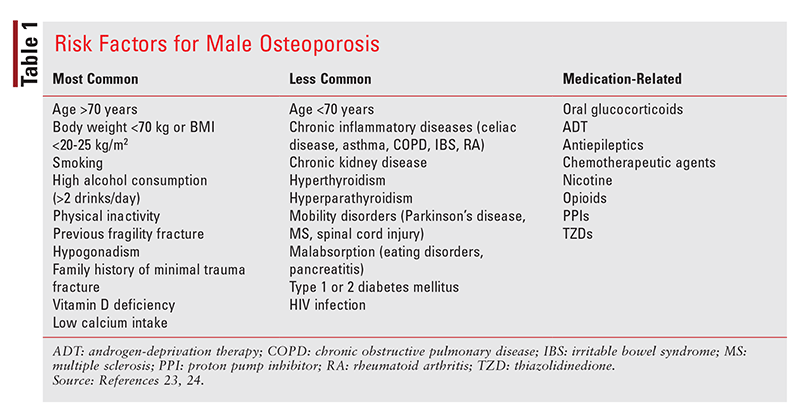
On the whole, primary causes of osteoporosis relate to the natural aging process and to inherent risks such as genetic polymorphisms and ethnicity, with non-Hispanic white and Asian individuals being particularly susceptible.25,26 In women, primary causes are based on age and are classified as either type 1 (also called postmenopausal osteoporosis; usually occurs 15-20 years post menopause) or type 2 (also known as senile osteoporosis; usually develops after age 70 years).27 In men, however, age 70 years should be used as a benchmark. The comparatively delayed bone loss observed in men versus women is generally due to slower rates of decline for testosterone and estradiol.4,28 This phenomenon is illustrated by the temporal profile of peak fracture incidence, which occurs approximately 10 years later in males.24
Secondary causes of male osteoporosis include sedentary lifestyle, poor nutrition, and medications, along with their associated disease states.29 Because of their impairment of androgen synthesis, oral glucocorticoids and ADT are of greatest concern.23 Through various pathophysiological events, many other agents—including TH, TZDs (glitazones, other peroxisome proliferator-activated receptor gamma agonists), antiepileptics, nicotine, SSRIs, anticoagulants, chemotherapeutics, immunosuppressants (e.g., cyclosporin A), and PPIs—induce BMD loss by promoting osteoblast apoptosis, osteoclastogenesis, hypogonadism, renal calcium wasting, decreased intestinal calcium absorption, mineral malabsorption (e.g., magnesium), or vitamin B12 deficiency.8-18
Diagnosis and Classification
Dual-energy x-ray absorptiometry (DEXA) scanning of the hip and spine is the gold standard for diagnosing BMD. DEXA scores are typically reported as T-scores and Z-scores.30 The T-score compares the patient’s BMD with that of a healthy 30-year-old of the same sex, whereas the Z-score compares it with that of an average person of identical age and sex.30 World Health Organization guidelines classify a patient’s BMD according to four diagnostic categories based on T-score relative to the reference mean BMD and T-score for young adults: 1) normal bone mass (BMD within 1 standard deviation [SD]); 2) low bone mass (BMD >1.0-<2.5 SDs below); 3) osteoporosis (BMD 2.5 or more SDs below); and 4) severe or established osteoporosis (BMD >2.5 SDs below in the presence of 1 or more fractures).5 BMD tests are commonly combined with more cost-effective, questionnaire-based risk-assessment tools such as the Fracture Risk Assessment Tool (FRAX) and QFracture.31 These calculation tools determine fracture risk via algorithms that factor several clinical-risk variables, including age, sex, weight, height, BMD, previous fracture history, family history of hip fracture, tobacco and alcohol intake, presence of rheumatoid arthritis, and secondary conditions related to osteoporosis.32
In addition to BMD testing, the following are key diagnostic tools for assessing risk: serum levels of 25-hydroxyvitamin D, calcium, TH, parathyroid hormone (PTH), and testosterone and bone-turnover markers (bone formation: bone-specific alkaline phosphatase, osteocalcin; bone resorption: telopeptides of type 1 collagen).33,34
According to the National Osteoporosis Foundation, male patients with a previous hip or vertebral fracture (clinical or asymptomatic) or a T-score −2.5 or less at the femoral neck, total hip, or lumbar spine should be offered treatment for osteoporosis regardless of age. Men should also be treated if they are aged 50 years or older and have low bone mass (T-score between −1 and −2.5 at femoral neck, total hip, or lumbar spine and a 10-year hip-fracture probability of 3% or more or 10-year major osteoporotic fracture [spine, hip, forearm, humerus] probability of 20% or more per FRAX).35
Management
Pharmacotherapy for male osteoporosis includes oral or IV bisphosphonates and other antiresorptive agents, PTH analogues, anabolic agents, testosterone replacement, vitamin D supplementation, and other investigational therapies, such as selective estrogen receptor modulators (SERMs).22,24 One key counseling point for pharmacists regarding risk reduction and management of osteoporosis in men is to explain the effectiveness of lifestyle modifications for preventing bone loss.
Bisphosphonates: The American College of Physicians recommends bisphosphonates as first-line treatment for reducing the risk of vertebral fracture in men with clinically recognized osteoporosis.36 In male patients, the number needed to treat with bisphosphonates to prevent one vertebral fracture is 16 over a 2-year period.37 Oral agents in this class include alendronate, ibandronate, and risedronate. These antiresorptive drugs slow bone breakdown by inhibiting proliferation of osteoclasts, decreasing their activity, and reducing their life span.38 The drug is preferentially incorporated into sites of active bone remodeling, where it inhibits breakdown of hydroxyapatite, a major mineral component of bone.38
In dysregulated bone metabolism, increased activity of extracellular adenosine triphosphate (ATP), activation of the mevalonate pathway, and pyrophosphate-induced impairments of mineralization all favor bone resorption over bone formation.38 Bisphosphonates are structural mimics of pyrophosphate wherein the P-O-P oxygen is replaced by carbon. This modification creates a nonhydrolyzable backbone and a much more stable molecule. The carbon also serves as a point of substitution, allowing for much variability in bisphosphonate agents. Like pyrophosphate, bisphosphonates bind to hydroxyapatite with high affinity. Whereas non–nitrogen-containing first-generation agents displace the terminal pyrophosphate of ATP within osteoclasts, nitrogen-containing second- and third-generation agents disrupt the mevalonate pathway.38
Bisphosphonates can cause adverse gastrointestinal (GI) effects, including esophagitis, dysphagia, abdominal pain, diarrhea, upset stomach, and heartburn. The dose should be taken immediately upon waking and at least 30 to 60 minutes before food or drink (except plain water), and the patient must remain upright for at least 30 to 60 minutes and until after the first food of the day is consumed in order to reduce the risk of esophageal irritation. One member of this class, zoledronic acid, is administered IV in patients who cannot take oral bisphosphonates because of GI issues or in whom adherence is a concern. Individual agents’ package inserts provide more detailed information on dosing and monitoring parameters.39-42
PTH Analogues: Although two members of this class are available for use in the U.S., only one—teriparatide—is FDA approved for use in males. Teriparatide, an anabolic drug that helps build bone, may be used for primary or hypogonadal osteoporosis or as an alternative agent for glucocorticoid-induced osteoporosis.36 Human PTH, an 84-amino-acid peptide, is the primary regulator of calcium and phosphorus metabolism in the bone and kidney.43 The parathyroid gland secretes PTH in response to hypocalcemia to directly increase renal tubular calcium reabsorption and to indirectly enhance intestinal calcium absorption by increasing circulating calcitriol levels.43 Teriparatide is a recombinant form of PTH composed of only the first 34 amino-acid residues, which constitute the bioactive sequence.43 Whereas chronically elevated PTH depletes and demineralizes bone, intermittent PTH exposure stimulates bone formation and mineralization because of increased osteoblast activity over osteoclast.43 Teriparatide is administered as a once-daily SC injection of 20 mcg into the thigh or abdominal wall, with a cumulative lifetime duration of therapy not to exceed 2 years.36,44
RANKL Inhibitors: The receptor activator of nuclear factor-kappa B ligand of the RANK receptor (RANKL) is expressed by osteoclasts and their precursors. The interaction between RANK and RANKL modulates bone breakdown via stimulatory effects on osteoclast differentiation, proliferation, and survival.45 Osteoprotegerin (OPG), also known as osteoclastogenesis inhibitory factor, is the natural inhibitor of RANKL and is primarily expressed by osteoblast-lineage cells under the regulatory control of estrogens.46 In animal models, OPG deficiency leads to osteoporosis, whereas overexpression results in elevated bone mass.45 Denosumab is a monoclonal antibody inhibitor (MAOI) of RANKL that antagonizes RANKL activity in a manner similar to that of OPG, thus exhibiting antiresorptive effects.45 It is indicated for use as an alternative agent in males with ADT-induced osteoporosis, glucocorticoid-induced osteoporosis, or primary osteoporosis, especially those who have failed or cannot tolerate bisphosphonates. Denosumab is administered by a healthcare professional (HCP) as a single-dose SC injection of 60 mg into the upper arm, upper thigh, or abdomen every 6 months. Duration of therapy is typically 5 to 10 years, and treatment carries an associated risk of jaw osteonecrosis and atypical fractures.36,47
Sclerostin Inhibitors: Romosozumab-aqqg, the newest agent in the armamentarium for osteoporosis, gained FDA approval for use in postmenopausal women with high fracture risk.48 However, evidence is limited on its safe and effective use in men with a previous fracture who have failed or cannot otherwise use other agents.49 This agent is unique in that it is both antiresorptive and anabolic, working to simultaneously build bone and slow bone breakdown. Sclerostin, which is secreted exclusively by osteocytes of mature bone, inhibits the primary metabolic pathway responsible for maintaining bone mass (i.e., osteoblast activity).50,51 Accordingly, osteocytes reduce the release of sclerostin in response to mechanical or weight-bearing stimuli acting on bone.51 Romosozumab-aqqg is an MAOI of sclerostin that augments the maintenance of bone mass.51 It is administered by an HCP as two consecutive injections (105 mg each) once monthly in the abdomen, thigh, or outer area of the upper arm. Therapy duration is limited to 12 months because the anabolic effects tend to wane thereafter. Romosozumab-aqqg carries a black box warning for potential risk of myocardial infarction (MI), stroke, and cardiovascular (CV) death; therefore, this agent should be avoided in patients who have had an MI or stroke within the past 12 months. The risks and benefits of therapy should be weighed in patients with significant CV risk factors.36,48
Sex-Hormone Replacement: Loss of estrogens and testosterone and other androgens in elderly men contributes to the development of osteoporosis, as these hormones play a critical role in bone formation during development and bone maintenance and remodeling in adults. Androgen deficiency promotes an increase in remodeling characterized by an imbalance between resorption and formation in which osteoclasts’ life span is increased and osteoblasts’ life span is decreased.52 Although testosterone therapy in men with osteoporosis has not been clearly defined, its use in testosterone deficiency increases bone density.53
Vitamin D Supplementation: The primary role of vitamin D in skeletal health is to facilitate the absorption of dietary calcium and phosphorus. Vitamin D, in the form of calcitriol, acts as a hormone that ultimately serves as a transcription factor for the production of calbindins.54 Calbindins are calcium-binding proteins that are especially important in the intestinal mucosa for the absorption and transport of dietary calcium and phosphorus. Vitamin D deficiency involves decreased calbindin production and, therefore, reduced calcium absorption. This state leads to increased osteoclast production and mobilization of calcium from bone.55 If hypocalcemia persists, PTH is released to increase renal reabsorption of calcium and to further stimulate osteoclast production for even more mobilization of bone calcium.
Investigational Therapies: SERMs are standard therapy for female osteoporosis, most notably raloxifene. Although these agents have not been adopted in male patients, results of some small-scale studies suggest the possibility of their use. For instance, raloxifene increases BMD of the hip in men receiving ADT for prostate cancer.56 Similar to SERMs, selective androgen receptor modulators (SARMs) have been investigated for treating muscle wasting and bone loss.57 To date, however, no SARM agents have been approved for use.
Lifestyle Modifications: Adequate intake of calcium (1,200-1,500 mg/day) and vitamin D (800-2,000 IU/day) through the diet, supplementation, or both; resistance exercise (i.e., weight-bearing exercise or strength training) at least three times per week; refraining from smoking and drinking excessive alcohol; and fall-prevention strategies (e.g., balance exercises) all serve to strengthen bone in order to prevent or delay loss.5
Conclusion
Despite all that is known about the risk factors, primary and secondary causes, incidence rates, and adverse outcomes following a high-trauma fracture, as well as the proven efficacy and general safety of multiple treatment options, osteoporosis continues to be underdiagnosed and undertreated in older men. Men are less likely to be evaluated and undergo treatment following a low-trauma fracture, which increases their risk of sustaining subsequent fractures.20 Treatment rates remain low even after high-trauma fracture involving the hip.20 Therefore, it is important for the pharmacist to foster increased awareness of male osteoporosis in the community. Identifying high-risk patients with comorbidities that share etiology or risk factors is a critical part of achieving earlier diagnosis of osteoporosis and ensuring its targeted management. Pharmacists serve as critical frontline HCPs who can advocate for regular bone-health screening in susceptible male patients and provide counsel on the benefits of leading a healthy lifestyle to prevent or delay bone loss and improve overall quality of life.
REFERENCES
1. Föger-Samwald U, Dovjak P, Azizi-Semrad U, et al. Osteoporosis: pathophysiology and therapeutic options. EXCLI J. 2020;19:1017-1037.
2. Veronese N, Kolk H, Maggi S. Epidemiology of fragility fractures and social impact. In: Falaschi P, Marsh D, eds. Orthogeriatrics: The Management of Older Patients with Fragility Fractures [Internet]. Cham, Switzerland: Springer; 2021.
3. Alswat KA. Gender disparities in osteoporosis. J Clin Med Res. 2017;9:382-387.
4. Shigehara K, Izumi K, Kadono Y, Mizokami A. Testosterone and bone health in men: a narrative review. J Clin Med. 2021;10:530.
5. Ebeling PR. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
6. McKenna MJ, Murray B. Vitamin D deficiency. In: Bandeira F, Gharib H, Golbert A, et al, eds. Endocrinology and Diabetes: A Problem-Oriented Approach. New York, NY: Springer; 2014:293-304.
7. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77:453-468.
8. Tuchendler D, Bolanowski M. The influence of thyroid dysfunction on bone metabolism. Thyroid Res. 2014;7:12.
9. McDonough AK, Rosenthal RS, Cao X, Saag KG. The effect of thiazolidinediones on BMD and osteoporosis. Nat Clin Pract Endocrinol Metab. 2008;4:507-513.
10. Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015;1:e000014.
11. Sansone RA, Sansone LA. SSRIs: bad to the bone? Innov Clin Neurosci. 2012;9:42-47.
12. Signorelli SS, Scuto S, Marino E, et al. Anticoagulants and osteoporosis. Int J Mol Sci. 2019;20:5275.
13. Adler RA. Management of osteoporosis in men on androgen deprivation therapy. Maturitas. 2011;68:143-147.
14. Guise TA. Bone loss and fracture risk associated with cancer therapy. Oncologist. 2006;11:1121-1131.
15. Maalouf NM, Shane E. Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab. 2005;90:2456-2465.
16. Gross RA, Gidal BE, Pack AM. Patient page. Antiseizure drugs and reduced bone density. Neurology. 2004;62:E24-E25.
17. Thong BK, Ima-Nirwana S, Chin KY. Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int J Environ Res Public Health. 2019;16:1571.
18. Spencer H, Kramer L. Osteoporosis: calcium, fluoride, and aluminum interactions. J Am Coll Nutr. 1985;4:121-128.
19. Stewart TL, Ralston SH. Role of genetic factors in the pathogenesis of osteoporosis. J Endocrinol. 2000;166:235-245.
20. Adler RA. Update on osteoporosis in men. Best Pract Res Clin Endocrinol Metab. 2018;32:759-772.
21. Endo Y, Aharonoff GB, Zuckerman JD, et al. Gender differences in patients with hip fracture: a greater risk of morbidity and mortality in men. J Orthop Trauma. 2005;19:29-35.
22. Jeremiah MP, Unwin BK, Greenawald MH, Casiano VE. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92:261-268.
23. Adler RA. Osteoporosis in men: a review. Bone Research. 2014;2:14001.
24. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
25. Leslie WD. Ethnic differences in bone mass—clinical implications. J Clin Endocrinol Metab. 2012;97:4329-4340.
26. Ralston SH. Genetics of osteoporosis: symposium on ‘genetic polymorphisms and disease risk.’ Proc Nutr Soc. 2007;66:158-165.
27. Riggs BL, Melton LJ III. Involutional osteoporosis. N Engl J Med. 1986;314:1676-1686.
28. Noirrit-Esclassan E, Valera MC, Trémollieres F, et al. Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical implications. Int J Mol Sci. 2021;22:1568.
29. Sapre S, Thakur R. Lifestyle and dietary factors determine age at natural menopause. J Midlife Health. 2021;5:3-5.
30. Dimai HP. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T- and Z-score, and reference databases. Bone. 2017;104:39-43.
31. Aw D, Thain J, Ali A, et al. Predicting fracture risk in osteoporosis: the use of fracture prediction tools in an osteoporosis clinic population. Postgrad Med J. 2016;92:267-270.
32. Gadam RK, Schlauch K, Izuora KE. Frax prediction without BMD for assessment of osteoporotic fracture risk. Endocr Pract. 2013;19:780-784.
33. Adler RA. Osteoporosis in men: insights for the clinician. Ther Adv Musculoskelet Dis. 2011;3:191-200.
34. Shetty S, Kapoor N, Bondu JD, et al. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20:846-852.
35. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
36. Clinical resource #361008. Managing osteoporosis: screening, treatment, and more. Pharmacist’s Letter/Prescriber’s Letter. October 2020.
37. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
38. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
39. Fosamax (alendronate) package insert. Whitehouse Station, NJ; Merck & Co, Inc; August 2019.
40. Boniva (ibandronate) package insert. South San Francisco, CA: Genentech USA, Inc; April 2020.
41. Actonel (risedronate) package insert. Madison, NJ: Allergan USA, Inc; November 2019.
42. Zometa (zoledronic acid) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; December 2018.
43. Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688-703.
44. Forteo (teriparatide) package insert. Indianapolis, IN: Lilly USA, LLC; November 2020.
45. McClung M. Role of RANKL inhibition in osteoporosis. Arthritis Res Therapy. 2007;9(suppl 1):S3.
46. Michael H, Härkönen PL, Väänänen HK, Hentunen TA. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res. 2005;20:2224-2232.
47. Prolia (denosumab) package insert. Thousand Oaks, CA: Amgen Inc; May 2021.
48. Evenity (romosozumab-aqqg) package insert. Thousand Oaks, CA: Amgen Inc; April 2020.
49. Lewiecki EM, Blicharski T, Goemaere S, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103:3183-3193.
50. Pietrzyk B, Smertka M, Chudek J. Sclerostin: intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med. 2017;26:1283-1291.
51. Glass DA II, Karsenty G. Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway. Curr Top Dev Biol. 2006;73:43-84.
52. Almeida M, Laurent MR, Dubois V, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97:135-187.
53. Vanderschueren D, Vandenput VL, Boonen S, et al. Androgens and bone. Endocr Rev. 2004;25:389-425.
54. Feher JJ, Fullmer CS, Wasserman RH. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am J Physiol. 1992;262:C517-C526.
55. Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clinic Risk Manag. 2008;4:827-836.
56. Kastelan D, Giljevic Z, Kraljevic I, Korsic M. Selective estrogen receptor modulators: a possible new treatment of osteoporosis in males. Med Hypotheses. 2006;67:1052-1053.
57. Komrakova M, Nagel J, Hoffmann DB, et al. Effect of selective androgen receptor modulator enobosarm on bone healing in a rat model for aged male osteoporosis. Calcif Tissue Int. 2020;107:593-602.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





