US Pharm. 2021;46(2):21-24.
ABSTRACT: Valvular heart disease (VHD) is a disorder in which a heart valve is damaged or diseased, resulting in poor cardiac blood flow. Types of VHD include aortic stenosis, aortic regurgitation, mitral stenosis, mitral regurgitation, and tricuspid regurgitation. Most patients with VHD require surgery to repair or replace the valve and subsequent antithrombotic therapy to prevent clotting. Antithrombotic therapy options that are commonly used after valve replacement or repair are vitamin K antagonists, such as warfarin, and antiplatelet agents, such as aspirin. Patient counseling points should include drug-drug or drug-food interactions, frequency of international normalized ratio monitoring with warfarin, and signs and symptoms of bleeding. Pharmacists are in an ideal position to partake in the management and care of patients with VHD.
Valvular heart disease (VHD) is a condition in which any one of the four heart valves is damaged or defective, resulting in improper blood flow throughout the heart. The etiology may be congenital or acquired. In the year 2000, a population series showed that the prevalence of moderate or severe VHD in the United States was estimated to be 2.5% on a systematic echocardiographic examination.1 Risk factors for VHD include increased age, male gender, tobacco use, hypercholesterolemia, rheumatic heart disease, and hypertension.2,3 In particular, VHD is especially prevalent in those older than age 75 years due to its potential degenerative etiology.4 Types of VHD include aortic stenosis, aortic regurgitation, mitral stenosis, mitral regurgitation, and tricuspid regurgitation. Chronic VHD can significantly decrease a patient’s quality of life and result in distressing symptoms, such as exertional dyspnea or angina. Most patients with VHD require surgery to repair or replace the valve. After a prosthetic valve replacement, optimal antithrombotic therapy is recommended to prevent thromboembolism while minimizing the risk of bleeding.
Etiology and Pathophysiology
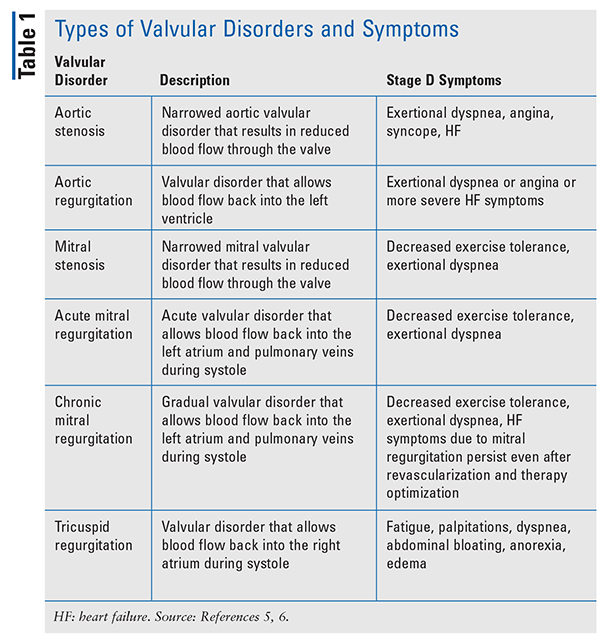
VHD can be a consequence of genetic defects or acquired etiology. This condition may be acquired through calcific aortic valve disease, which can range from mild valve thickening to severe calcification and impairment of valve movement.2 Stages of VHD range from stage A, where factors for developing VHD are identified, to stage D, where a patient is experiencing more advanced symptoms.5 As VHD progresses, stage D symptoms, such as decreased exercise tolerance and exertional dyspnea, are observed among people with the various valvular disorders. Refer to TABLE 1 for the types of valvular disorders and their associated stage D symptoms.5,6
Treatment Approach
The therapeutic goals for treating VHD include reducing symptoms, repairing or replacing valves, and preventing blood clots. Beta-blockers and other antihypertensive agents can be used to improve symptoms associated with VHD.5 However, most patients will require surgical intervention, such as valvular repair or replacement. After the procedure, it is recommended that patients be started on antithrombotic therapy, such as warfarin or aspirin, to prevent thromboembolism.5 These patients are at higher risk of clotting post-surgery because turbulent blood flow around the valves results in high shear stresses, which can lead to platelet activation on the valve surface.7 The choice of an antithrombotic agent is dependent upon the location of the replaced valve and the type of valve replacement(s).
Types of Valve Replacements
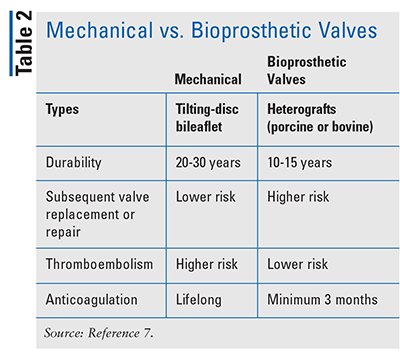
The type of valve replacement a patient receives is dependent on several factors, includingbut not limited to age, life expectancy, risk of anticoagulation complications, and patient preference.8 A comparison of the types of prosthetic valves is shown in TABLE 2. Mechanical prosthesis is preferred in younger patients (age 50 years or younger) while bioprosthesis is preferred in patients age 70 years or older.8 A bioprosthetic valve is also preferred in patients who have a contraindication to anticoagulation therapy or in patients unable to commit to the lifelong anticoagulation that is required with a mechanical prosthetic valve.8 When selecting a valve, patients who prefer to avoid the possibility of another valve procedure in the future may choose a mechanical valve, because its durability is longer than that of a bioprosthetic valve.7,8
When determining the method by which the valve replacement is performed, a transcatheter aortic valve replacement (TAVR) is indicated for patients with severe aortic stenosis who have an intermediate or high risk for aortic valve replacement and a predicted postprocedure survival longer than 1 year. This procedure involves the use of catheters to direct a nonnative aortic valve into the position of the native aortic valve.9 Prior to implementation of the less-invasive TAVR procedure, surgical aortic valve replacements (SAVRs) were used in patients with severe symptomatic aortic stenosis.10 SAVR is an open-heart procedure in which the damaged aortic valve is removed and replaced with a new valve.11 The choice between TAVR and SAVR depends on candidate operability, valve durability, and patient survival, as trials have demonstrated the noninferiority of TAVR compared with SAVR.10,12 However, some compelling indications for SAVR include, but are not limited to, patients younger than age 65 years who will likely need a subsequent valve replacement; high risk for a permanent pacemaker; and rheumatic heart disease.10
PHARMACOLOGIC THERAPY
Antithrombotic Therapy for Valve Replacement
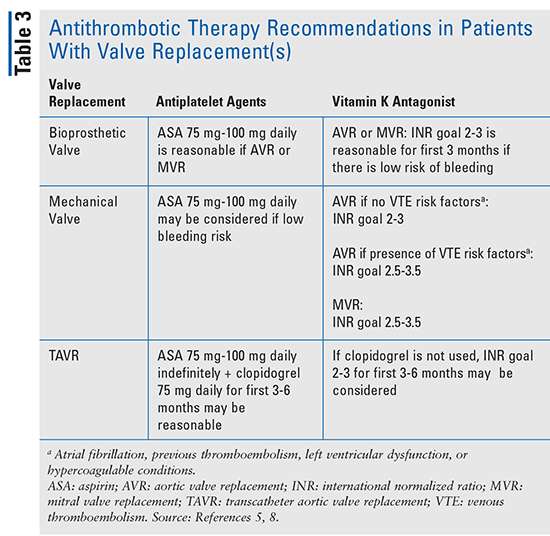
After valve replacement(s), antithrombotic therapy with an antiplatelet and vitamin K antagonist (VKA) is recommended with the dosing, duration, and international normalized ratio (INR) goal varying among bioprosthetic and mechanical replacements and TAVR. Administering daily low-dose aspirin (75 mg to 100 mg) indefinitely and a VKA to obtain an INR goal of 2-3 for the first 3 months is reasonable for patients with a bioprosthetic valve who have a low risk of bleeding.
It is recommended that patients with mechanical valves be on daily low-dose aspirin indefinitely and a VKA, but the INR goal depends on the type of mechanical valve and the presence of venous thromboembolism (VTE) risk factors. If the patient does not have VTE risk factors, such as atrial fibrillation, previous VTE, left ventricular dysfunction, or hypercoagulable conditions, and has a bileaflet or current-generation single tilting disc aortic valve replacement (AVR), the recommended INR goal is 2-3. However, the INR goal is higher at 2.5-3.5 for patients with VTE risk factors or an older-generation mechanical AVR, since this group has an increased risk of clotting.5
The use of direct oral anticoagulants (DOACs) has not been sufficiently studied nor approved by the FDA for prophylaxis or treatment of prosthetic-valve thrombosis.8 In patients with mechanical heart valves, DOACs are not recommended, and dabigatran is specifically contraindicated.5,13 A study published in 2013 comparing dabigatran and warfarin in patients with mechanical heart valves showed that patients taking dabigatran were at a higher risk of thromboembolic and bleeding complications than those taking warfarin.13
For patients post-TAVR, daily low-dose aspirin indefinitely and clopidogrel 75 mg daily for the first 3-6 months may be reasonable.5 Antiplatelet therapy is preferred over DOACs in these patients, as rivaroxaban 10 mg daily has been shown to put patients who are not indicated for anticoagulation for another reason at a higher risk of death or thromboembolic complications compared with antiplatelet therapy.14 Refer to TABLE 3 for a summary of the antithrombotic therapy recommendations for patients with valve replacement(s).
Atrial Fibrillation
In patients with atrial fibrillation and native aortic valve disease, tricuspid valve disease, or mitral regurgitation who have undergone a prosthetic valve replacement, DOACs can be used as an alternative to VKA.8 However, for patients with atrial fibrillation and mitral stenosis who have undergone a prosthetic valve replacement, anticoagulation with a VKA is indicated. It is important to note that DOACs are not recommended in mitral stenosis.8 Rivaroxaban 20 mg once daily was found to be noninferior to dose-adjusted warfarin with a target INR of 2-3 in patients with atrial fibrillation and a bioprosthetic mitral valve. The primary outcome measured was a composite of death, major cardiovascular events, or major bleeding at 1 year.15
Bridging for Surgical Procedures
Patients with prosthetic valves are at high risk of clotting, so continuation of antithrombotic therapy may be recommended even in patients undergoing surgical procedures.8 However, the increased risk of bleeding must be considered. For minor procedures, including dental extractions or cataract removal, continuing warfarin to reach a therapeutic INR is recommended for patients with mechanical heart valves.5 For invasive procedures, even when the INR is subtherapeutic, it is recommended to hold the VKA without bridging for patients with a bileaflet mechanical AVR and no thrombotic risk factors.5 Based on the previously stated criteria, when the interruption of VKA therapy is recommended, the agent is typically held 3 to 4 days preprocedure to allow the INR to fall to an adequate level during the procedure. However, bridging with either IV unfractionated heparin (UFH) or SC low-molecular-weight heparin (LMWH) is recommended in patients undergoing invasive or surgical procedures and who meet any of the following criteria: mechanical AVR and any VTE risk factor, older-generation mechanical AVR, or mechanical mitral valve replacement. Bridging is usually started 36 to 48 hours before surgery and stopped 4 to 6 hours for UFH and 12 hours for LMWH before the procedure. The VKA is usually restarted 12 to 24 hours postoperatively as bleeding risk allows.8
Pregnancy
Antithrombotic therapy recommendations for pregnant patients differ from those of nonpregnant patients. Pregnant patients with mechanical valves should be on anticoagulation therapy unless contraindicated. Although warfarin is normally contraindicated in pregnancy due to its potential teratogenic effects, it is acceptable to use in patients with mechanical valve(s) in all three trimesters of pregnancy due to the lack of an appropriate alternative. In the first trimester, it is reasonable to continue warfarin to maintain a therapeutic INR if the dose is 5 mg per day or less. However, the benefits and risks of using warfarin during pregnancy should be discussed with the patient. If warfarin 5 mg per day is insufficient, LMWH or UFH can be used as an alternative to reach a therapeutic anti-Xa level or activated partial thromboplastin time (aPTT), respectively. For the second and third trimesters, warfarin is recommended at any dose to achieve a therapeutic INR. Daily low-dose aspirin is also recommended in the second and third trimesters. Discontinuation of warfarin and initiation of LMWH or UFH is recommended at least 1 week prior to planned vaginal birth.5
Role of the Pharmacist
Pharmacists have the opportunity to play a key role in the management and monitoring of antithrombotic therapy for patients with VHD. Pharmacists can educate patients about their disease state and the significance of adherence to their medications as well as provide recommendations to physicians on appropriate occasions. As warfarin is a high-risk medication, pharmacists can take additional precautions to counsel on the following: frequent INR monitoring, minor and major bleeding signs, potential drug-drug interactions, and consuming a consistent amount of dietary vitamin K. Common medications that interact with warfarin include nonsteroidal anti-inflammatory drugs, antiplatelet agents, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors due to an additive bleeding risk and amiodarone, azole antifungals, metronidazole, and sulfamethoxazole-trimethoprim as they may increase INR. In addition to identifying common drug-drug interactions, pharmacists can also educate patients on symptoms of VHD progression as well as emphasize the importance of following up with their physician. Through these clinical interventions, pharmacists can contribute to the safe and effective care for patients with VHD.
The content in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-1011.
2. Zeng YI, Sun R, Li X, et al. Pathophysiology of valvular heart disease. Exp Ther Med. 2016;11:1184-1188.
3. Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630-634.
4. Ray S. Changing epidemiology and natural history of valvular heart disease. Clin Med Lond. 2010;10:168-171.
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. December 17, 2020. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000923 [online ahead of print}. Accessed January 25, 2021.
6. Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85:483-500.
7. Tillquist MN, Maddox TM. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer Adherence. 2011;5:91-99.
8. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
9. Alfadhli J, Jeraq M, Singh V, Martinez C. Updates on transcatheter aortic valve replacement: techniques, complications, outcome, and prognosis. J Saudi Heart Assoc. 2018;30:340-348.
10. Still S, Szerlip M, Mack M. TAVR vs. SAVR in intermediate-risk patients: what influences our choice of therapy. Curr Cardiol Rep. 2018;20:82.
11. Ramlawi B, Ramchandani M, Reardon MJ. Surgical approaches to aortic valve replacement and repair—insights and challenges. Interv Cardiol. 2014;9:32-36.
12. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
13. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214.
14. Dangas GD, Tijssen JGP, Wöhrle J, et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med. 2020;382:120-129.
15. Guimarães HP, Lopes RD, de Barros E Silva PGM, et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med. 2020;383:2117-2126.
To comment on this article, contact rdavidson@uspharmacist.com.