US Pharm. 2010;35(10):52-56.
Diabetes affects more than 23.6 million people in the United States. Although believed to be underreported, this metabolic disorder is the seventh leading cause of death, with mortality occurring at a rate nearly twice that of individuals without the disease.1 Mortality is attributed to macrovascular complications such as peripheral vascular disease, cardiovascular disease (CVD), and stroke.1
Background
In 1997, the American Diabetes Association (ADA) issued its initial secondary-prevention recommendation to use aspirin in adults with diabetes and coexisting CVD. The ADA further proposed that high-risk patients over 30 years of age with diabetes and without CVD receive antiplatelet therapy as primary prevention.2 Subsequently, for more than a decade, the ADA has recommended aspirin as a standard of medical care, although the primary-prevention age criterion was further refined in 2004 to patients over 40 years of age.3 Based on evolving clinical evidence, the ADA further modified primary-prevention aspirin recommendations in 2010 to encompass only patients with a 10-year CV risk greater than 10%.4
The 2010 ADA standards for medical care in diabetes demonstrate the continued shift toward practicing evidence-based medicine.4 Previous ADA recommendations were based on older trials that involved small numbers of patients with diabetes and failed to show clear evidence of aspirin’s efficacy in primary prevention.4,5 The increased risk of significant adverse effects of aspirin therapy (e.g., gastrointestinal [GI] bleeding, hemorrhagic stroke), coupled with the lack of evidence of clinical efficacy, highlights the uncertainty of aspirin’s net value for primary prevention in patients with diabetes.
As noted above, the revised recommendation is to consider aspirin treatment as a primary prevention strategy in patients with diabetes who are at increased CV risk (defined as a 10-year risk greater than 10%). Patients at increased CV risk include men older than 50 years and women older than 60 years with at least one additional major risk factor (TABLE 1).4 The basis for the updated recommendation for aspirin use for primary prevention is two recent randomized, controlled trials of aspirin in patients with diabetes, a meta-analysis conducted by the Antithrombotic Trialists’ (ATT) Collaboration, and evidence-based recommendations from the U.S. Preventive Services Task Force (USPSTF).

Clinical Trials
Prevention of Progression of Arterial Disease and Diabetes (POPADAD) Trial: The POPADAD trial was a randomized, multicenter, double-blind, placebo-controlled, two-by-two factorial-design study assessing whether aspirin (100 mg) and antioxidant therapy (vitamin E 200 mg and ascorbic acid 100 mg, along with trace elements), combined or alone, were more effective than placebo in reducing the development of CV events in patients with diabetes and asymptomatic peripheral arterial disease.6 Between November 1997 and July 2001, 1,276 eligible subjects aged 40 years and over with diabetes and an ankle-brachial pressure index of 0.99 or less were randomized to receive aspirin tablets plus antioxidant capsules (n = 320), aspirin tablets plus placebo capsules (n = 318), placebo tablets plus antioxidant capsules (n = 320), and placebo tablets plus placebo capsules (n = 318). Median length of follow-up was 6.7 years, and final follow-up was in 2006.
Results after final follow-up showed no significant reduction in the primary endpoint (death from coronary heart disease [CHD], nonfatal myocardial infarction [MI] or stroke, or above-ankle amputation for critical limb ischemia) with either aspirin (18.2% for aspirin vs. 18.3% for placebo) or antioxidant (18.3% for antioxidant vs. 18.2% for placebo). No significant difference in specific adverse events was found between the aspirin and placebo groups.6 See TABLE 2.6
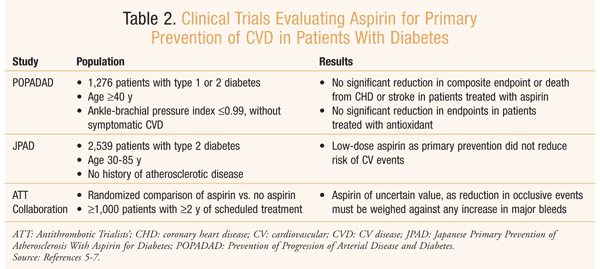
Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial: The JPAD trial was a prospective, randomized, open-label, blinded, endpoint-assessment study designed to examine the efficacy of low-dose aspirin for primary prevention of any atherosclerotic event in patients with type 2 diabetes.7 The trial was conducted from December 2002 through April 2008 at 163 institutions throughout Japan, enrolling 2,539 patients aged 30 to 85 years with type 2 diabetes and no history of atherosclerotic disease. Of these, 1,262 patients were randomized to the low-dose aspirin group (81 mg or 100 mg) and 1,277 were assigned to the nonaspirin group. Follow-up visits were scheduled every 2 weeks for patients seen in a clinic setting and every 4 weeks for those seen in a hospital setting, with a median follow-up of 4.37 years.
A total of 154 atherosclerotic events occurred, 68 in the aspirin group and 86 in the nonaspirin group. Incidence of the primary endpoint of any atherosclerotic event was not significantly different between the aspirin group (68 events, 5.4%) and the nonaspirin group (86 events, 6.7%). The authors concluded that, in patients with type 2 diabetes without previous CVD, low-dose aspirin as primary prevention did not reduce the risk of CV events.7 However, because of the low incidence of atherosclerotic disease in Japan, this finding may not be applicable to the North American population.8 See TABLE 2.7
ATT Collaboration Report: Results of a meta-analysis conducted to assess the benefits and risks of low-dose aspirin for primary prevention of vascular disease were published in 2009.5 The ATT Collaboration analyzed six prospective primary prevention trials, each of which included at least 1,000 participants without a history of occlusive disease and in which patients were randomized to receive aspirin or no aspirin for at least 2 years. Data on 95,000 patients (approximately 4,000 with diabetes) were utilized to evaluate serious vascular events (MI, stroke, or vascular death) and major bleeds.
Compared with patients who received no aspirin, those taking aspirin had a statistically significant, but very small, absolute reduction (0.07% per year) and a 12% proportional reduction in serious vascular events (0.51% for aspirin vs. 0.57% for control per year, P = .0001). This was due mainly to a reduction of nonfatal MI (0.18% vs. 0.23% per year, P <.0001). However, aspirin allocation had no significant effect on stroke (0.20% vs. 0.21% per year; hemorrhagic stroke, 0.04% vs. 0.03%; other stroke, 0.16% vs. 0.18% per year), vascular mortality (0.19% vs. 0.19% per year), or total mortality. Patients taking aspirin experienced significantly more major GI and extracranial bleeds (0.10% vs. 0.07% annually, relative risk 1.54 [95% CI 1.30-1.82, P <.0001]). It was concluded that, in the primary prevention of CVD, aspirin is of uncertain net clinical value, as the reduction in occlusive events needs to be weighed against any increase in major bleeds.5 See TABLE 2.5
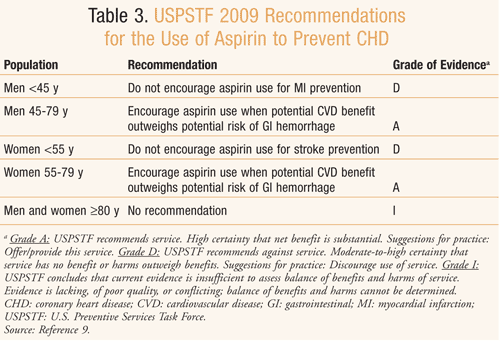
USPSTF Recommendations: An update of the 2002 USPSTF recommendations regarding the use of aspirin for the prevention of CHD was published in 2009 (TABLE 3).9 The USPSTF is an independent panel of private-sector experts in prevention and primary care whose recommendations are considered the gold standard for clinical preventive services. Its latest recommendations were made after the panel reviewed the literature published since 2002, focusing on new evidence of the benefits and harms of aspirin for the primary prevention of CVD, including MI and stroke. The benefit of aspirin, in the panel’s opinion, depended upon the initial risk for CHD events versus the potential harm from its use (e.g., side effects such as GI bleeding, hemorrhagic stroke). Initial risk assessment for CHD included diabetes, age, total cholesterol levels, HDL cholesterol levels, blood pressure, and smoking.
Conclusion
Low-dose aspirin remains a secondary prevention strategy in patients with diabetes and a history of CVD, yet it is no longer advocated as primary prevention for all patients aged 40 years and older with diabetes. Low-dose aspirin is now recommended for patients with an increased CV risk (10-year risk >10%), including men older than 50 years and women older than 60 years with at least one additional major risk factor.4 Ongoing clinical trials involving patients with diabetes, such as A Study of Cardiovascular Events in Diabetes and the Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes, may further elucidate the role of aspirin use for the primary prevention of CV events.10,11
REFERENCES
1. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008.
2. American Diabetes Association. Aspirin therapy in diabetes. Diabetes Care. 1997;20:1772-1773.
3. American Diabetes Association. Aspirin therapy in diabetes. Diabetes Care. 2004;27(suppl 1):S72-S73.
4. American Diabetes Association. Standards for medical care in diabetes—2010. Diabetes Care.
5. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
6. Belch J, MacCuish A, Campbell I, et al. The Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.
7. Ogawa H, Nakayama M, Morimoto T, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134-2141.
8. Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702-2709.
9. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396-404.
10. Krantz MJ, Berger JS, Hiatt WR. An aspirin a day: are we barking up the wrong willow tree? Pharmacotherapy. 2010;30:115-118.
11. De Berardis G, Sacco M, Evangelista V, et al. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials. 2007;8:21.
To comment on this article, contact rdavidson@uspharmacist.com.





