US Pharm. 2010;35(11):HS-9-HS-15.
A behavioral emergency--also known as clinically significant acute agitation--typically is a dynamic, unpredictable encounter with a patient in whom the diagnosis is unknown or preliminary. Class I studies are limited or nonexistent, possibly because of the emergent need for treatment in highly agitated patients.1,2 Several lower-level class II and class III studies have been conducted.2-16 It is for this reason that the Expert Consensus Guidelines were developed. The guidelines were formulated based on responses to a statistically designed written survey that was administered to 48 experts in the field.1 Clinically significant acute agitation that required emergency intervention was defined as involving “explosive and/or unpredictable anger; intimidating behavior; restlessness, pacing, or excessive movement; physical and/or verbal self-abusiveness; demeaning or hostile verbal behavior; uncooperative or demanding behavior or resistance to care; and impulsive or impatient behavior or low tolerance to pain or frustration.”1
Expert Consensus Guidelines
The treatment of choice endorsed by the Expert Consensus Guidelines is to calm the patient without sedation.1 Second-line treatment is mild sedation to the point of drowsiness, but not sleep; third-line treatment is heavy sedation or sleep. In general, the recommendations are based on a preliminary diagnosis. Clinically significant acute agitation should be treated with an agent that will effectively treat the underlying condition.1
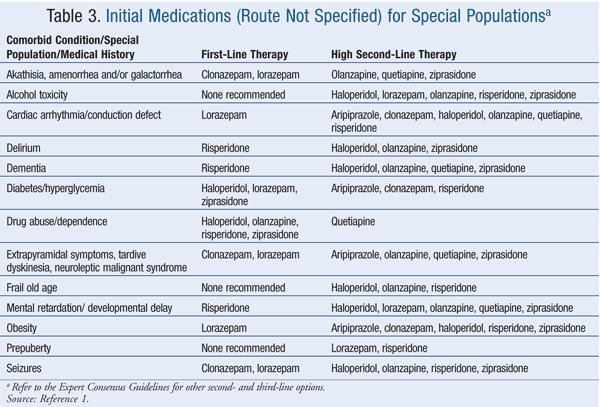
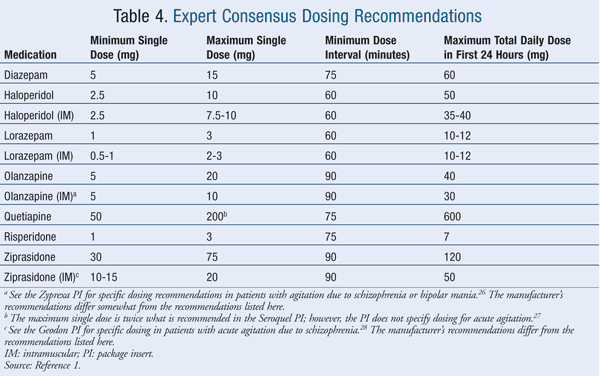
Historically, lorazepam and haloperidol have been used most often for treating clinically significant acute agitation.17 Of the agents listed in the Expert Consensus Guidelines, 100% of survey respondents considered intramuscular (IM) and oral formulations of lorazepam and haloperidol to be appropriate.1 Thirty-eight percent of respondents considered diazepam to be inappropriate for emergency treatment, followed by oral ziprasidone (31% of respondents), oral quetiapine (25%), IM ziprasidone (9%), IM olanzapine (2%), and oral risperidone (2%).1 TABLES 1 and 2 list the appropriate first- and high second-line oral and parenteral agents, respectively, while TABLE 3 gives appropriate choices for special patient populations. TABLE 4 describes the dosing specified by the consensus survey. Refer to the Expert Consensus Guidelines for additional recommendations regarding failure of initial treatment.1
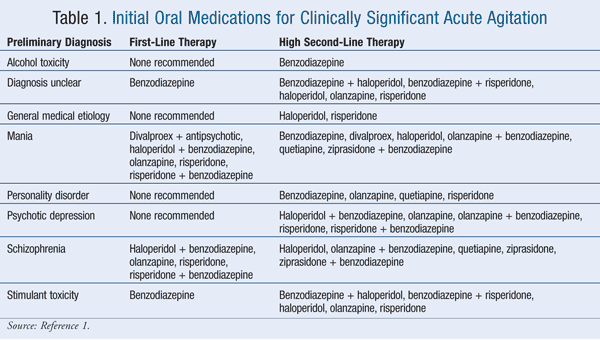
Within the limits of the expert opinion, the Consensus Guidelines suggest that benzodiazepines are first-line therapy when there is no clear diagnosis, when there is stimulant intoxication, and when parenteral therapy is needed for a personality disorder.1 Benzodiazepines are a high second-line option if medication must be used for alcohol intoxication.1 Lorazepam is recommended as first-line therapy if the following comorbid conditions exist: cardiac arrhythmia or conduction defect; obesity; or diabetes or hyperglycemia.1 Haloperidol and ziprasidone are also recommended as first-line therapy for diabetes or hyperglycemia.1 Lorazepam and clonazepam are first-line therapy if the patient has a history of akathisia, amenorrhea and/or galactorrhea, seizures, extrapyramidal symptoms, tardive dyskinesia, or neuroleptic malignant syndrome.1 Risperidone is recommended as first-line therapy for delirium, dementia, mental retardation, and drug abuse or dependence.1 Olanzapine, haloperidol, and ziprasidone are recommended as first-line agents for drug abuse or dependence.1
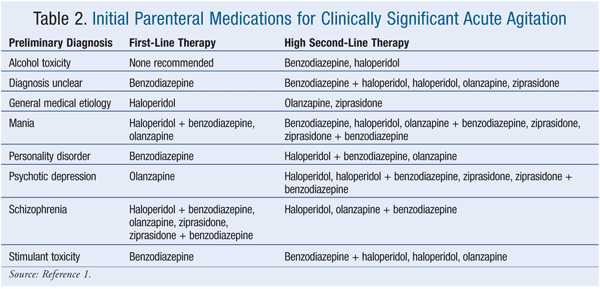
It is unclear whether combination therapy with a benzodiazepine and an antipsychotic confers greater efficacy, a more rapid onset of action, or reduced side effects. The literature does not support the use of combination therapy to lower the dose of each medication.1 However, within the limits of the expert opinion, the Consensus Guidelines suggest that second-generation antipsychotics, alone or in combination with a benzodiazepine, are first-line or high second-line treatment when acute agitation is the result of a primary psychiatric condition such as schizophrenia, mania, or psychotic depression.1
The clinical policy of the American College of Emergency Physicians (ACEP) differs from that of the Expert Consensus Guidelines in that the ACEP recommends droperidol as an agent of choice and suggests that “rapid sedation” may also be obtained with droperidol.2 The Expert Consensus Guidelines do not recommend the use of droperidol for the treatment of behavioral emergencies even if the expert panel did not consider any risk with droperidol use.1 The expert panel, in contrast to the ACEP, strongly endorsed the goal of emergency treatment to “calm the patient without sedation.”1
At the time of the survey, the expert panel did not have a consensus on the use of IM aripiprazole, and the ACEP did not address the use of aripiprazole; therefore, the dosing is not listed in TABLE 4.1 Studies in patients with acute agitation who have Alzheimer's disease, vascular or mixed dementia, bipolar disorder, or schizophrenia indicate that aripiprazole is effective and well tolerated.18-20 The single IM aripiprazole dose in these studies was 9.75 mg to 15 mg. Although there was no consensus on the use of aripiprazole, those experts who used aripiprazole recommended a minimum single dose of approximately 11 mg, a maximum single dose of approximately 25.5 mg, a minimum wait-time interval between doses of 75 minutes, and a maximum total dose in the first 24 hours of approximately 31.50 mg.1
Medication Safety
Important safety concerns with the use of medications used to treat clinically significant acute agitation include the potential for drug interactions, cardiotoxic effects, cerebrovascular effects, and hypotension. When a clinician is deciding whether to administer these agents acutely with the intent to begin long-term therapy, the effects that they may have on blood glucose and lipid profiles should be considered.21 Torsades de pointes has been reported with IV haloperidol at high oral doses (420-1,000 mg).21 However, definitive cases have not been reported with risperidone, olanzapine, or ziprasidone even though a change in the QTc may be seen with atypical antipsychotics. A black box warning exists for aripiprazole, clozapine, olanzapine, quetiapine, risperidone, and ziprasidone. The warning states that there is an “increased risk of death and increased incidence of cerebrovascular adverse events” in elderly patients with dementia-related psychosis.22
Hypotension may be seen with lorazepam at low levels. Haloperidol alone does not appear to confer a significant risk of hypotension, but severe hypotension can occur with haloperidol in combination with antihypertensives, leading in rare cases to cardiac arrest. Other side effects seen with use of haloperidol and other atypical antipsychotics in the acute setting may include extrapyramidal symptoms, akathisia, and dystonic reactions.17
Important Drug Interactions
Risperidone and Olanzapine: These drugs are metabolized via CYP2D6. There may be an increased effect with either agent when strong inhibitors of CYP2D6 (fluoxetine, paroxetine, high-dose sertraline) are used concomitantly.23 Olanzapine is metabolized primarily via CYP1A2, and it may result in a decreased response if it is administered to a patient who smokes cigarettes.23
Ziprasidone and Quetiapine: These agents are metabolized via CYP3A4. Agents that induce CYP3A4 (carbamazepine, phenytoin) or inhibit it (ketoconazole, protease inhibitors) can affect ziprasidone and quetiapine concentrations.23
Aripiprazole: Aripiprazole is metabolized via CYP3A4 and CYP2D6. Adjustments should be made when enzyme inducers or inhibitors are used concomitantly.24
Haloperidol: Significant interactions can be seen with the administration of haloperidol plus carbamazepine, lithium, or serotonin reuptake inhibitors.25
Conclusion
The primary goal in treating patients with clinically significant acute agitation is to calm the patient without sedation. This can facilitate the patient-physician relationship and aid in proper diagnosis of the underlying disease, thus allowing for initiation of appropriate first-line therapy. Haloperidol is still recommended as a first-line or high second-line choice in many cases, and the benzodiazepine most widely recommended by the Expert Consensus Guidelines is lorazepam. The guidelines recommend second-generation antipsychotics alone or in combination with a benzodiazepine as first-line or high second-line therapy when acute agitation is the result of a primary psychiatric condition such as schizophrenia, mania, or psychotic depression. Data are inconclusive concerning greater efficacy, more rapid onset of action, or reduced side effects with the use of combination therapy. The literature does not support using combination therapy to lower the dose of each medication.
REFERENCES
1. Allen MH, Currier GW, Carpenter D, et al. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(suppl 1):5-112.
2. Lukens TW, Wolf SJ, Edlow JA, et al. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47:79-99.
3. Salzman C, Solomon D, Miyawaki E, et al. Parenteral lorazepam versus parenteral haloperidol for the control of psychotic disruptive behavior. J Clin Psychiatry. 1991;52:177-180.
4. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med.
5. Foster S, Kessel J, Berman ME, et al. Efficacy of lorazepam and haloperidol for rapid tranquilization in a psychiatric emergency room setting. Int Clin Psychopharmacol. 1997;12:175-179.
6. Nobay F, Simon BC, Levitt MA, Dresden GM. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11:744-749.
7. Thomas H, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21:407-413.
8. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16:567-573.
9. Harrigan EP, Miceli JJ, Anziano R, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62-69.
10. Daniel DG, Potkin SG, Reeves KR, et al. Intramuscular (IM) ziprasidone 20 mg is effective in reducing acute agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl). 2001;155:128-134.
11. Lesem MD, Zajecka JM, Swift RH, et al. Intramuscular ziprasidone, 2 mg versus 10 mg, in the short-term management of agitated psychotic patients. J Clin Psychiatry. 2001;62:12-18.
12. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry. 2002;59:441-448.
13. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158:1149-1151.
14. Meehan K, Zhang F, David S, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21:389-397.
15. Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology. 2002;26:494-504.
16. Currier GW, Chou JC, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65:386-394.
17. Battaglia J. Pharmacological management of acute agitation. Drugs. 2005;65:1207-1222.
18. Rappaport SA, Marcus RN, Manos G, et al. A randomized, double-blind, placebo-controlled tolerability study of intramuscular aripiprazole in acutely agitated patients with Alzheimer's, vascular, or mixed dementia. J Am Med Dir Assoc. 2009;10:21-27.
19. Zimbroff DL, Marcus RN, Manos G, et al. Management of acute agitation in patients with bipolar disorder: efficacy and safety of intramuscular aripiprazole. J Clin Psychopharmacol. 2007;27:171-176.
20. Tran-Johnson TK, Sack DA, Marcus RN, et al. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:111-119.
21. Alexopoulos GS, Streim J, Carpenter D, Docherty JP. Expert consensus guidelines for using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):100-102.
22. Rosack J. FDA orders new warning on atypical antipsychotics. Psychiatr News. 2005;40:1.
23. Sharif ZA. Pharmacokinetics, metabolism, and drug-drug interactions of atypical antipsychotics in special populations. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 6):22-25.
24. Abilify (aripiprazole) product information. Rockville, MD: Otsuka America Pharmaceutical, Inc; November 2009.
25. Haloperidol. Facts & Comparisons [by subscription]. http://online. 1997;15:335-340.
26. Zyprexa (olanzapine) product information. Indianapolis, IN: Eli Lilly and Co; May 2010.
27. Seroquel (quetiapine fumarate) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; May 2010.
28. Geodon (ziprasidone) product information. New York, NY: Pfizer Inc; November 2009.
To comment on this article, contact rdavidson@uspharmacist.com.






