US Pharm. 2015;40(2):HS11-HS15.
ABSTRACT: Recent data demonstrate an increase in emergency department visits necessitated by illicit drug use. Cocaine is a highly addictive illegal substance that produces a euphoric effect. Adverse effects, primarily cardiovascular in nature, are of concern because of their severe and possibly chronic nature. Cocaine-induced cardiomyopathy, one of these effects, may require pharmacologic or interventional therapy. Decisions regarding initiation of therapy and patient follow-up post exposure are determined based on the patient’s initial presentation and the clinical picture observed during the recovery period. The role of the pharmacist is to ensure that pharmacologic treatment has been properly identified and implemented and to counsel patients on the importance of abstinence from cocaine use.
Of the more than 2.5 million emergency department visits related to drug abuse in the United States in 2011, more than half resulted from illicit drug use.1 Approximately 40% of these visits were related to cocaine use, which indicates that cocaine is perhaps one of the most commonly used illicit drugs in the U.S.1 In 2012, 2.2% of the U.S. population aged 12 years and older used cocaine.2
Background
Cocaine (benzoylmethylecgonine) is a highly addictive crystalline tropine alkaloid derived from the coca plant, which is native to South America.3 The two forms of cocaine are the hydrochloride salt form, which is commonly injected or sniffed, and its freebase formulation (also known as “crack” or “crack cocaine”), which is typically smoked. Cocaine produces physiological responses ranging from euphoric effects to cardiovascular complications.
With cocaine use, stimulation of the sympathetic nervous system results in blockage of catecholamine reuptake at the sympathetic nerve terminals.3 This inhibition excites central nervous system sympathetic outflow and increases the adrenergic nerve endings’ sensitivity to norepinephrine.3 Euphoric effects are caused by the modulation of neurotransmitter activity, particularly an increase in dopamine in the brain’s mesolimbic region.4 The potency and duration of the euphoric effects are contingent upon the route of administration. Because of the lack of absorption with IV administration, injection of cocaine has a more rapid effect than other routes of administration (e.g., oral ingestion, nasal inhalation). Absorption rates for nasal inhalation and oral ingestion are similar; however, faster rates have been observed with nasal inhalation.4
Inhalation results in a more rapid onset and a shorter duration of action compared with ingestion.4 The half-life of cocaine depends upon its route of administration and varies from 1 minute up to 2 hours.4 Cholinesterase, an enzyme found in the liver and the blood, metabolizes cocaine into inactive water-soluble metabolites of benzoylecgonine and ecgonine methyl ester.4 These by-products, which are excreted primarily via urination, remain detectable in the urine or bloodstream for up to 72 hours.4
A multitude of health consequences, particularly adverse cardiovascular events, are likely to result from the use of cocaine. Cocaine exerts effects on endothelial cells by stimulating the release of endothelin-1, a potent vasoconstrictor, and inhibiting the production of nitric oxide, a major vasodilator.5 In addition, cocaine stimulates the cardiovascular system, increasing the heart rate and blood pressure by blocking the peripheral norepinephrine transporter.3 Cocaine use is associated with cardiovascular diseases including myocardial infarction (MI), arrhythmias, congestive heart failure (CHF), aortic dissection, endocarditis, and cardiomyopathy.6
Types of Cardiomyopathy
Cardiomyopathy is a condition in which the heart muscles become thickened and enlarged. In advanced cases, the myocardium is weakened and can no longer support adequate organ perfusion.7 The four types of cardiomyopathy are dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy, and arrhythmogenic right ventricular dysplasia. Each type is summarized in TABLE 1.7,8
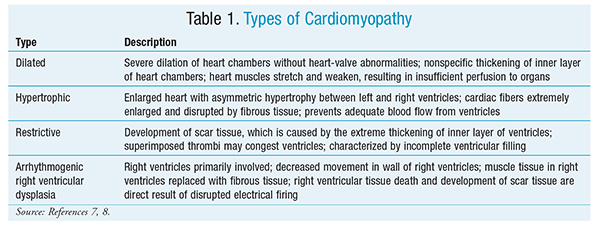
Dilated cardiomyopathy, the most common type, can lead to several complications, such as heart failure, arrhythmias, and heart-valve deficiencies.7 In hypertrophic cardiomyopathy, the heart muscles thicken, but the ventricles remain normal in size.7,8 The thickness of the lower chambers prevents blood from flowing out of the ventricles; the lower chambers compensate by working harder, resulting in symptoms such as chest pain, shortness of breath, and dizziness.7 Complications of this physiological change may lead to valve leakage and arrhythmias.7 Restrictive cardiomyopathy is characterized by the presence of scar tissue caused by extreme thickening of the endocardium and can lead to heart failure and arrhythmias.7,8 Arrhythmogenic right ventricular dysplasia, which is rare, confers the risk of arrhythmias and palpitations.7
Acute and Chronic Presentation
Cocaine-induced cardiomyopathy (CIC) results from a deprivation of myocardial oxygen supply coupled with an increased demand for oxygen, which leads to a reduction in coronary blood flow. Deterioration of myocardial performance is a concern because a worsening of this situation leads to atherosclerosis and, ultimately, cardiomyopathy.7
Cocaine decreases myocardial contraction. A lessening of coronary capacity and blood flow induces electrical irregularities in the heart, causing increased heart rate and blood pressure.9 Acute intoxication may cause nonischemic myocardial depression, leading to dilated cardiomyopathy.10 A subtype of cardiomyopathy, Takotsubo cardiomyopathy (“broken-heart syndrome”), has been associated with acute cocaine use. Takotsubo cardiomyopathy presents abruptly, causing a substantial increase in catecholamines and, therefore, injury and dysfunction within the heart.10 Normal cardiac function is expected to return immediately upon cessation of cocaine intake.
Case reports and autopsies of chronic cocaine users confirm dilated cardiomyopathy as the most common type of CIC.11-13 Catecholamine toxicity associated with chronic use leads to an increase in the activity of cytotoxic natural killer cells, causing myocarditis.14 This is significant because myocarditis is known to cause dilated cardiomyopathy. Clinical manifestations of CIC are similar to those of dilated cardiomyopathy unrelated to cocaine use.14
Drug-Drug Interactions
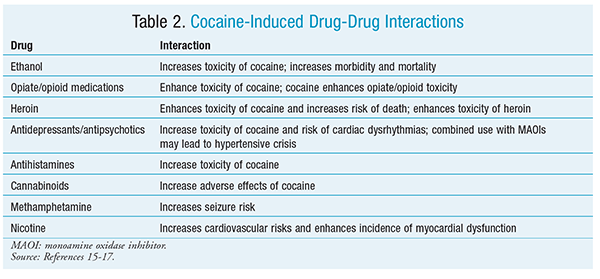
Most cocaine addicts concurrently abuse other illicit drugs. Substances such as ethanol, opioids, heroin, and antidepressants, to name a few, have well-documented drug interactions with cocaine (TABLE 2).15-17
Pharmacologic Treatment
Management of CIC is similar to that of non-CIC.18 Initial therapy includes pharmacologic interventions targeting patient-specific acute presentation (TABLE 3).19-23 Benzodiazepines (BZDs) are the preferred therapy because they counteract the adrenergic effects seen in cocaine use.24 Nitrates and calcium channel blockers (CCBs) are recommended as second-line and third-line agents, respectively, in cases of unresolved counteractivity despite BZD initiation.19
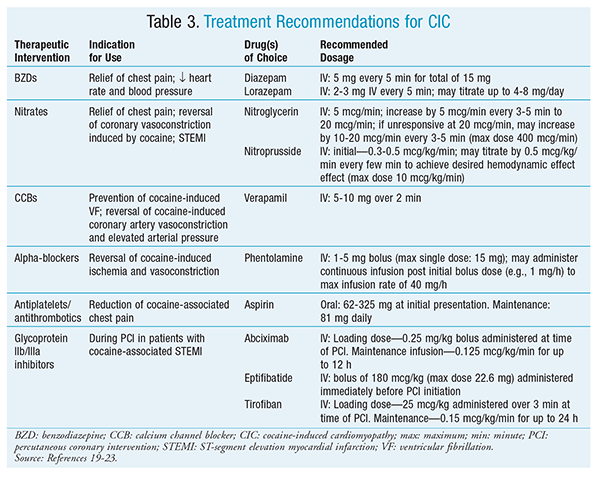
Verapamil is the CCB of choice. A clinical trial in humans demonstrated that verapamil successfully reversed cocaine-induced coronary artery vasoconstriction and elevation in arterial pressure.20 Chest pain may be treated with aspirin, nitroglycerin, or nitroprusside.19 Aspirin should be continued indefinitely in patients with MI or coronary artery disease.19 If non–ST-segment elevation MI (non-STEMI) is present, the patient may receive IV nitroglycerin.25 Additionally, phentolamine has shown some benefit in managing cocaine-induced ischemia.19
In the presence of STEMI, percutaneous coronary intervention (PCI) should be performed.25 The use of glycoprotein IIb/IIIa inhibitors should be recommended during PCI. Treatment of CHF and pulmonary edema induced by CIC consists of standard treatment regimens, with the exception of beta-blocker (BB) use.18
BBs should be avoided as first-line treatment in the management of the acute phase of CIC.26 The alpha-adrenergic receptors stimulated by cocaine are unopposed in the presence of BBs, leading to increases in adrenergic receptor activity. Onset or exacerbation of existing coronary vasoconstriction and hypertension are probable.26
Pharmacist’s Role
The role of the pharmacist is to recognize patient-specific presentations and ensure that pharmacologic treatment has been properly identified and implemented. The primary goal of therapy is to resolve the symptoms caused by the onset of cocaine use. BZDs, nitrates, CCBs, and several other agents play a role in the management of CIC. BBs should be avoided in the acute management of CIC. To prevent further cardiac deterioration, it is advisable that the pharmacist counsel patients on the importance of abstinence from cocaine use, as well as consult with all members of the patient’s healthcare team.27
Conclusion
Cocaine use is likely to result in adverse cardiovascular events. One of these events, cardiomyopathy, takes various forms, the most common type being dilated cardiomyopathy. Cessation of cocaine use reduces acute symptoms of CIC and could reverse chronic CIC.
REFERENCES
1. Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
2. National Institute on Drug Abuse. National survey of drug use and health. www.drugabuse.gov/national-survey-drug-use-health. Accessed September 5, 2014.
3. Vongpatanasin W, Mansour Y, Chavoshan B, et al. Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation. 1999;100:497-502.
4. Jeffcoat AR, Perez-Reyes M, Hill JM, et al. Cocaine disposition in humans after intravenous injection, nasal insufflation (snorting), or smoking. Drug Metab Dispos. 1989;17:153-159.
5. Wilbert-Lampen U, Seliger C, Zilker T, Arendt RM. Cocaine increases the endothelial release of immunoreactive endothelin and its concentrations in human plasma and urine: reversal by coincubation with sigma-receptor antagonists. Circulation. 1998;98:385-390.
6. Goldfrank LR, Hoffman RS. The cardiovascular effects of cocaine. Ann Emerg Med. 1991;20:165-175.
7. National Heart, Lung, and Blood Institute. What is cardiomyopathy? www.nhlbi.nih.gov/health/health-topics/topics/cm. Accessed September 2, 2014.
8. Olsen EG. Pathological recognition of cardiomyopathy. Postgrad Med J. 1975;51:277-281.
9. Kazimir M. Cocaine-related cardiomyopathy. Medscape. http://emedicine.medscape.com/article/152535-overview. Accessed September 1, 2014.
10. Restrepo CS, Rojas CA, Martinez S, et al. Cardiovascular complications of cocaine: imaging findings. Emerg Radiol. 2009;16:11-19.
11. Cooper CJ, Said S, Alkhateeb H, et al. Dilated cardiomyopathy secondary to chronic cocaine abuse: a case report. BMC Res Notes. 2013;6:536.
12. Virmani R, Robinowitz M, Smialek JE, Smyth DF. Cardiovascular effects of cocaine: an autopsy study of 40 patients. Am Heart J. 1988;115:1068-1076.
13. Felker GM, Hu W, Hare JM, et al. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore). 1999;78:270-283.
14. Egred M, Davis GK. Cocaine and the heart. Postgrad Med J. 2005;81:568-571.
15. Laizure SC, Parker RB. Pharmacodynamic evaluation of the cardiovascular effects after the coadministration of cocaine and ethanol. Drug Metab Dispos. 2009;37:310-314.
16. Molina DK, Hargrove VM. Fatal cocaine interactions: a review of cocaine-related deaths in Bexar County, Texas. Am J Forensic Med Pathol. 2011;32:71-77.
17. Knuepfer MM. Cardiovascular disorders associated with cocaine use: myths and truths. Pharmacol Ther. 2003;97:181-222.
18. Phillips K, Luk A, Soor GS, et al. Cocaine cardiotoxicity: a review of the pathophysiology, pathology, and treatment options. Am J Cardiovasc Drugs. 2009;9:177-196.
19. Schwartz B, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122:2558-2569.
20. Negus BH, Willard JE, Hillis LD, et al. Alleviation of cocaine-induced coronary vasoconstriction with intravenous verapamil. Am J Cardiol. 1994;73:510-513.
21. Merck Manual Professional Edition. Drug use and dependence: cocaine. www.merckmanuals.com/professional/special_subjects/drug_use_and_dependence/cocaine.html. Accessed September 30, 2014.
22. Lexi-Drugs Online [online database]. Hudson, OH: Lexi-Comp, Inc; 2014.
23. Baumann BM, Perrone J, Hornig SE, et al. Randomized, double-blind, placebo-controlled trial of diazepam, nitroglycerin, or both for treatment of patients with potential cocaine-associated acute coronary syndromes. Acad Emerg Med. 2000;7:878-885.
24. Honderick T, Williams D, Seaberg D, Wears R. A prospective, randomized, controlled trial of benzodiazepines and nitroglycerine or nitroglycerine alone in the treatment of cocaine-associated acute coronary syndromes. Am J Emerg Med. 2003;21:39-42.
25. Hollander JE. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
26. Lange RA, Cigarroa RG, Flores ED, et al. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann Intern Med. 1990;112:897-903.
27. Zacà V, Lunghetti S, Ballo P, et al. Recovery from cardiomyopathy after abstinence from cocaine. Lancet. 2007;369:1574.
To comment on this article, contact rdavidson@ushpharmacist.com.





