US Pharm. 2021;(46):36-42.
ABSTRACT: Mesenchymal epithelial transition (MET) alterations are noted in up to 5% of non–small-cell lung cancer (NSCLC) cases and can present as either a MET exon 14 skipping mutation or MET amplifications. MET-selective kinase inhibitors have become available for the treatment of NSCLC in recent years and have displayed remarkable efficacy in clinical studies to date. Capmatinib and tepotinib are both approved for the treatment of MET-dysregulated advanced NSCLC, and savolitinib and glumetinib are currently under investigation for their application in the MET-mutated environment.
Lung cancer is one of the deadliest malignancies in the United States today.1 In 2021, it is estimated that about 235,760 new cases of lung cancer will arise, and 131,880 patients will succumb to the disease.1 Lung cancer is primarily broken down into two subtypes, small-cell lung cancer and non–small-cell lung cancer (NSCLC). NSCLC is the more common subtype, which originates from the epithelial cells of the central bronchi to the terminal alveoli and can be further divided into different histological subtypes (squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma) depending on the site of oncogenesis.1 Within NSCLC, adenocarcinoma is the most common histology, often presenting with a driver mutation, such as epidermal growth factor receptor (EGFR); kirsten rat sarcoma (KRAS); anaplastic lymphoma kinase (ALK); v-raf murine sarcoma viral oncogene homolog B1 (BRAF); proto-oncogene tyrosine-protein kinase (ROS1); rearranged during transfection (RET); mesenchymal epithelial transition (MET); erythroblastic oncogene B (ERBB2) genes; and others.2
MET Mutations
One of the more recent genetic aberrations within NSCLC that has moved therapeutic developments forward in recent years lies within the MET gene.3 Initially identified as a conventionally expressed biological gene in the 1980s, MET dysregulation was first characterized in lung cancer in the 1990s.3 Located on chromosome 7q21-q31, the MET gene encodes for a transmembrane receptor tyrosine kinase that is activated by hepatocyte growth factor (HGF), inducing downstream intracellular signaling pathways which permit cell survival and growth.3 MET and its ligand HGF serve pivotal roles in mediating wound healing, hepatic regeneration, and regulation of embryonic, neural, and muscle development in normal pathological systems.4 When expressed in malignancies, however, MET mutations result in proliferation, cell growth, and invasiveness in a variety of neoplasms.3,4
In advanced NSCLC, MET mutations are typically seen in those aged 70 years or older, unlike other oncogenic drivers (EGFR, ALK, ROS1).5,6 Additionally, as opposed to other mutations within NSCLC, MET alterations may be present in all possible histologic subtypes, whereas most other mutations are found almost exclusively in adenocarcinomas.3 Possessing great value as a prognostic factor and tool for determination of responsiveness to treatment, MET mutations are traditionally tested in patients with advanced disease.3
In this article, two distinct forms of MET dysregulation will be reviewed, along with the existing approved and investigational agents for the treatment of them: the exon 14 skipping mutation (METex14), which has been well defined regarding its mechanism of pathogenesis and pharmacological target , and the amplification/overexpression of the gene, which is less understood to date.3,7 Instances of METex14 are mutually exclusive from other genetic mutations involved in NSCLC, while in contrast, gene amplifications are often seen in patients who harbor EGFR mutations, and they possess acquired resistance to anti-EGFR inhibitors; 5% to 20% of NSCLC cases harboring EGFR mutations with resistance to prior EGFR tyrosine kinase inhibitors (TKIs) exhibit MET-amplifications.3,5,7 METex14 and MET-amplifications are noted in 3% to 4% and 1% to 5% of NSCLC cases, respectively, varying based on the degree of preselection and assay used.3,7 Such findings have provided direction for the contemporary clinical development of MET-targeted TKIs in recent years.
The initial use of oncolytics to target MET mutations was established with the multikinase inhibitors cabozantinib and crizotinib, where early case reports and studies described a benefit in progression-free survival (PFS) and overall survival (OS) in the MET-mutated setting.8-12 The utility of such agents implies the potential for MET-selective agents, which may diminish adverse effects via off-target kinase inhibition, and increased activity through selective modulation of the tumor microenvironment. To date there are two TKIs approved for the treatment of metastatic NSCLC with METex14: capmatinib and tepotinib (TABLE 1). Other agents such as savolitinib (AZD6094) and glumetinib (SCC244) are currently in development.3
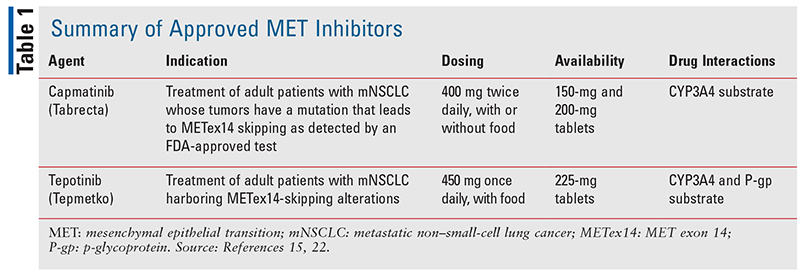
Capmatinib
Capmatinib was the first approved targeted MET-selective agent in NSCLC, based on results from the phase II, GEOMETRY mono-1 study (TABLE 2).13 In this study, 364 adult patients who presented with stage IIIB or IV NSCLC with METex14 or a MET- amplification, and without an EGFR mutation or ALK fusion, were assigned to two groups based on prior treatment status (either no previous treatment or having previously received one or two lines of therapy).13 These groups were further stratified to cohorts based on presence of METex14 or MET-amplification, and subdivided by gene copy number (GCN).9 In the patient group with prior treatments, five cohorts were included involving MET-amplified patients with GCN ³10, 6-9, 4-5, and £4, respectively, and patients with METex14 with any GCN.13 Similarly, in the patient group with no previous treatments, two cohorts were included involving MET-amplified patients with any GCN and patients with METex14 with any GCN.13 In the METex14 cohorts, 41% of those who were previously treated and 68% of those who were treatment naïve had a response to capmatinib.13 Additionally, the median duration of response among previously treated patients was 9.7 months (95% confidence interval [CI], 5.6-13.0) and 12.6 months (95% CI, 5.6–could not be estimated) among treatment-naïve patients.13 Notably, responses to capmatinib were rapid, as 82% of previously treated patients and 68% without prior treatment exhibited an initial response within the first cycle of therapy.13 In regard to subjects with MET-amplifications, response rates varied from 7% to 40% depending on GCN and prior treatment status.13
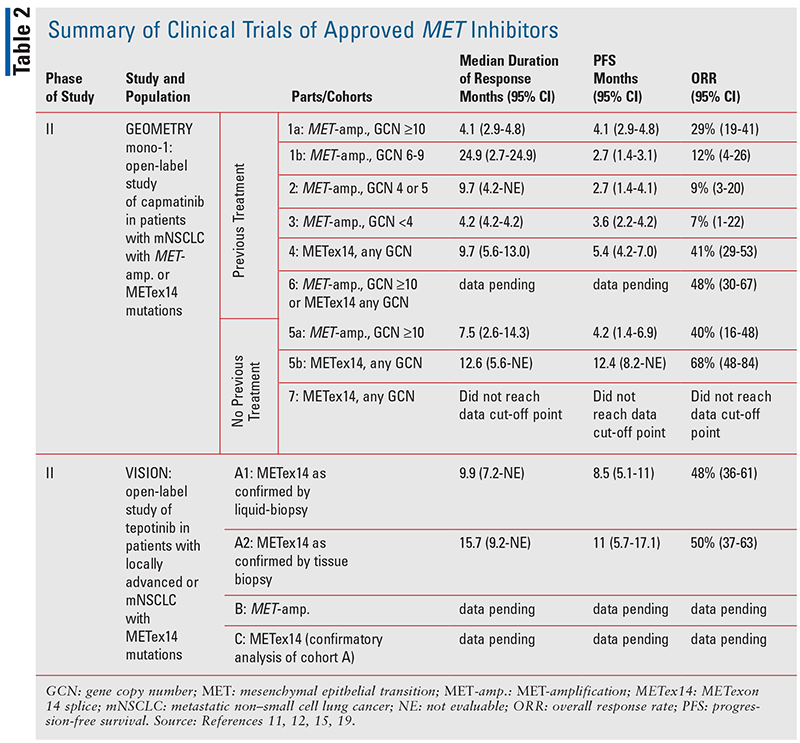
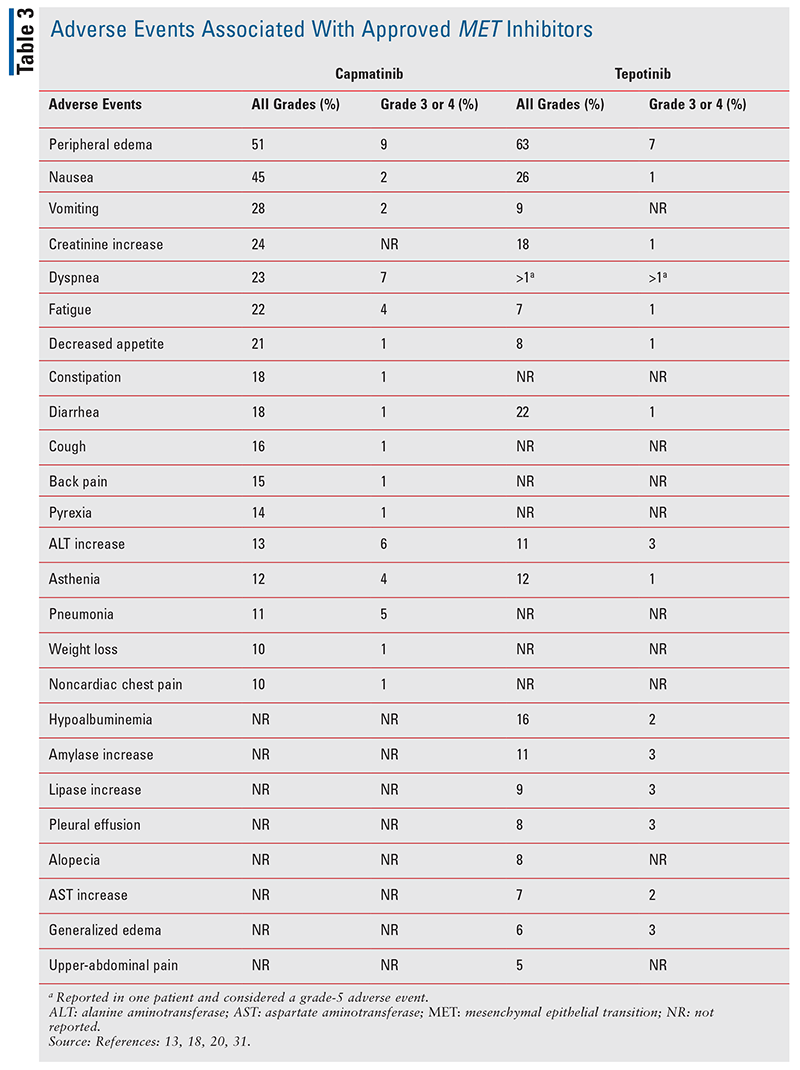
The efficacy of capmatinib in previously treated and treatment-naive MET-mutated NSCLC is encouraging, given the poor prognosis associated with MET dysregulation.13 Prior to the characterization and use of targeted agents, patients with MET mutations had limited therapeutic options.3 The GEOMETRY mono-1 study elucidated the clinical efficacy of capmatinib in the previously untreated METex14 setting, leading to its subsequent approval as the first targeted therapy in this mutational environment. Additionally, capmatinib displayed efficacy in patients with concomitant METex14 and brain metastases, as seven of 13 patients with brain involvement experienced responses.13 As capmatinib displayed nonconclusive efficacy in a majority of subjects with MET-amplifications, more studies will be needed to determine the utility of capmatinib in the MET-amplified setting. The most common adverse event noted was peripheral edema, which was observed in 54% of patients across all cohorts with 14% of patients experiencing grade 3 or 4 peripheral edema; TABLE 3 lists other common adverse events that occurred in 10% or more of patients.13 Further studies will describe the efficacy of capmatinib in combination with immunotherapy and in the setting of EGFR mutations with acquired resistance to anti-EGFR inhibitors.14-17
Tepotinib
Tepotinib is a highly selective MET-targeted TKI that has displayed efficacy in the METex14 environment.18 The initial utility of tepotinib was established in the VISION study, leading to its subsequent approval as the second MET-directed TKI (TABLE 2).18 This study was a phase II, open-label, multinational program that examined 99 patients with MET-mutated metastatic NSCLC for responses to tepotinib.18 Three cohorts were used in this study; patients with METex14 were entered in cohort A, patients with MET-amplification were entered in cohort B, and a subsequent group of patients with METex14 will be entered in cohort C for confirmatory analysis of cohort A, as recruitment is still ongoing.18 After at least 9 months of follow-up, 46% (95% CI, 36-57) of patients with METex14 were deemed to be responders according to independent review, although none were considered complete responders.18 The response rate as judged by investigator assessment was 56% (95% CI, 45-66), with two patients considered complete responders and 53 patients considered partial responders.14 Tumor shrinkage was noted in the majority of patients, and rapid responses were noted similar to capmatinib.18 Additionally, response rates were similar in both the treatment-naive and pretreated groups.19 Analyses of cohort B are still pending, although tumor-reduction sizes of at least 60% were noted in four of fivepatients with concomitant MET-amplification, suggesting strong potential for tepotinib in the MET-amplified setting.18
The VISION trial demonstrated tepotinib to be an efficacious agent in patients with metastatic NSCLC with METex14. The most common adverse event reported was peripheral edema, which was seen in 63% of patients, and other notable adverse events occurring in 10% or more of patients are listed in TABLE 3.18-20 A unique differentiating variable versus capmatinib is tepotinib’s utility as a once-daily dosing regimen, whereas capmatinib is dosed twice daily.18,19 Additionally, further studies will validate the efficacy of tepotinib in combination with the third-generation EGFR inhibitor osimertinib, in the osimertinib-resistant MET-mutated setting.18-19
Savolitinib
Savolitinib is an investigational MET-selective agent that has displayed pronounced efficacy in preclinical and clinical studies.21,22 Differing from the current FDA-approved MET-directed therapies, the clinical development strategy for savolitinib has been centered upon correction of MET-driven acquired resistance to anti-EGFR TKIs.21,22 EGFR mutations are one of the more common genetic aberrations present in NSCLC cases, and respond well to EGFR-selective TKIs.3,22,23 Though efficacious, targeted EGFR TKIs can eventually develop a variety of resistance mechanisms involving various ancillary microbiological pathways.23 One such resistance mechanism involves the presence of MET mutations, causing the formation of bypass tracks that enforce disease progression and mobilization via intracellular signaling.22 Emerging data have suggested that the combination of an EGFR inhibitor and MET-selective TKI possess the potential to prevent or correct MET-driven resistance to EGFR-selective TKIs.22 Though savolitinib has displayed single antitumor activity and tolerability in patient-derived xenografts and clinical testing, respectively, current studies have focused on its utility in acquired resistance to EGFR TKIs.22,24 This utility has thus far been studied in the TATTON study, which is a phase I, multinational, open-label investigation of the EGFR inhibitor osimertinib in combination with the mitogen-activated protein kinase (MEK) inhibitor selumetinib, the programmed death-ligand 1 inhibitor durvalumab, or savolitinib in patients who have progressed after an anti-EGFR therapy.22,23 As many secondary resistance mechanisms to osimertinib, including MET mutations, exist, the TATTON study is directed by concomitant use of agents that may enforce continuity of osimertinib therapy and rectify osimertinib resistance.21,22
Part A of the TATTON study consisted of a dose-ranging trial to elucidate a clinically potent yet safe regimen of savolitinib to be utilized.23 The findings from part A of the study determined savolitinib 600 mg once daily to be the preferred dosage for further analysis in part B.22 Substratification of part B included three prespecified cohorts of patients aged 18 years or older with MET-amplified, EGFR mutation-positive NSCLC; cohort B1 included patients who had received previous treatment with a third-generation EGFR TKI, whereas cohorts B2 and B3 included patients who had not received previous treatment with a third-generation EGFR TKI.22 Patients in B2 were EGFR T790M-negative at enrollment, whereas patients in B3 were T790M-positive at enrollment.22 A part D expansion cohort was later opened and consisted of patients with MET-amplified, EGFR mutation-positive NSCLC who had received previous treatment with a first- or second-generation EGFR TKI but had no previous history of a third- generation EGFR TKI and were EGFR T790M-negative at enrollment.22 Due to an identified safety signal of dose-related hypersensitivity, the protocol was later amended, causing patients in part B to receive savolitinib 300 mg if they weighed less than 55 kg, and savolitinib 600 mg if they weighed greater than 55 kg, while all patients in part D received savolitinib 300 mg.22
In part B, a total of 138 patients were evaluated.22 Sixty-six (48%; 95% CI, 39-56) patients experienced an objective response; 21 of 69 (30%; 95% CI, 20-43) in cohort B1, 33 of 51 (65%; 95% CI, 50-78) patients in cohort B2, and 12 of 18 (67%; 95% CI, 41-87) in cohort B3 displayed an objective response.22 All responses were considered partial responses, and across all patients in this cohort, the median PFS was 7.6 months (95% CI, 5.5-9.2).22 Similarly, in cohort D, 23 (64%; 95% CI, 46-79) patients had an objective response, with all being a partial response, and the median PFS was 9.1 months (95% CI, 5.4-12.9).22 Adverse events were noted frequently in both cohorts, as 83% and 60% of patients in part B and D, respectively, experienced adverse events attributed to savolitinib.22 Across both cohorts, side effects such as nausea (44%), decreased appetite (29%), fatigue (29%), peripheral edema (29%), vomiting (28%), and diarrhea (26%) were common.22 Seventy-nine of 138 (57%) patients in cohort B experienced a grade-3 or worse adverse event, most notably including leukopenia (3%), drug hypersensitivity (4%), alanine aminotransferase (ALT) increases (5%), aspartate aminotransferase (AST) increases (7%), neutropenia (4%), and Stevens-Johnson syndrome (1%).22 In cohort D, 16 of 42 (38%) patients experienced a grade-3 or worse adverse event, including pneumonia (12%), hypotension (5%), drug hypersensitivity (7%), pulmonary embolism (4%), and generalized edema (5%).22
MET-amplification is responsible for at least 5% of acquired resistances to first- and second-generation EGFR TKIs and up to 25% of acquired resistance to osimertinib (third-generation EGFR TKI).22 As osimertinib remains the preferred frontline treatment of EGFR-mutated NSCLC, these resistant patterns are significant.22 In the TATTON study, savolitinib offered explicit benefit in cohorts that did not receive a prior third-generation EGFR TKI, whereas patients who received a prior third-generation EGFR TKI had a notably lower rate of response.22 Since patients in the study who had received a prior third-generation EGFR TKI were generally heavily pretreated, the implications of the associated results from the TATTON program will be further investigated in the SAVANNAH trial, where the sequence of osimertinib to savolitinib + osimertinib with no other previous treatment will be studied.22 Nonetheless, the results of the TATTON program suggest that the combination of savolitinib + osimertinib possesses encouraging antitumor activity in the MET-amplified, EGFR mutation–positive setting.22 Further data are needed in order for savolitinib to become an approved anti-MET agent for the treatment of acquired anti-EGFR TKI resistance.22
Savolitinib has also been investigated for its capacity to treat pulmonary sarcomatoid carcinomas (PSC), a rare form of NSCLC that accounts for 0.3% to 3% of all cases of NSCLC and bears an overall 5-year survival rate of approximately 20%.25,26 PSC typically does not respond well to chemotherapy and has limited effective treatments.27,28 Pathologic investigations have revealed that cytogenic biomarkers exist within cases of PSC that bear potential for targeted pharmacologic activity, similar to conventional NSCLC.26,27 Prior clinical studies have reflected a prevalence of 9% to 22% of METex14 within PSC, varying based on patient populations and study design.25-27 As PSC generally does not respond well to conventional chemotherapy, MET-directed therapies propose an attractive alternative for therapy.26 In a phase II, multicenter, multicohort, single-arm study, savolitinib was evaluated in patients with concomitant PSC and METex14.26 Seventy-one adult patients with METex14 and unresectable or metastatic NSCLC were treated; they were stratified to receive savolitinib 600 mg for weight ³50 kg or 400 mg for weight <50 kg until disease progression or unacceptable toxicity.25 Fifty-seven percent and 36% of patients, respectively, presented with adenocarcinoma and PSC, with the remaining harboring other histologic subtypes.25 Additionally, 60% of patients had received prior treatments for NSCLC.25 The overall response rate (ORR) was 47.5% (95% CI, 34.6-60.7), disease-control rate was 93.4% (95% CI, 84.1-98.2), and the median duration of response had not yet been reached.24 The median PFS was 6.8 months (95% CI, 4.2-13.8) among all treated patients.25 Adverse events that presented at rates of ³20% included peripheral edema, nausea, AST and ALT increases, vomiting, and hypoalbuminemia; the incidence of grade-3 or more adverse events was 41.4%.25 In conclusion, savolitinib displayed promising efficacy and tolerability in PSC associated with METex14 and holds potential to become the first approved agent in this setting.25,28
Glumetinib
Glumetinib is a MET-targeted TKI currently undergoing both preclinical and early clinical trials.29,30 In xenografts and cell lines derived from tumor tissues with aberrant MET-regulation, glumetinib was found to have profound antiproliferative and antiangiogenic inhibitory effects.29 Clinical testing of glumetinib thus far has consisted of an open-label, dose-escalation, phase I study to determine its safety, efficacy, and antitumor effect in NSCLC patients regardless of MET mutational status.30 Although definitive data supporting the clinical use of glumetinib in the MET-altered setting are pending, early in-human studies have displayed its potential in metastatic NSCLC.30
Role of the Pharmacist
As the environment of MET-directed therapies is still nascent, an oncology pharmacist serves many pivotal capacities in managing adverse events and assessing treatment efficacy. Both FDA-approved agents, capmatinib and tepotinib, carry a risk of interstitial lung disease (ILD)/pneumonitis, as 4.5% and 2.2% of patients, respectively, experienced new or worsening dyspnea, cough, and/or fever.20,31 Though confirmatory data are pending, 2% to 3% of patients in phase I studies of savolitinib have also experienced ILD/pneumonitis.18 If any symptoms of ILD/pneumonitis are experienced, both capmatinib and tepotinib should be permanently discontinued.20,31 Additionally, capmatinib and tepotinib have both displayed risks for hepatotoxicity via increased ALT and AST.20,31 Depending on the severity of the reaction, capmatinib and tepotinib can be withheld, dose-reduced, or discontinued for appropriate management.20,31 For both agents, liver enzymes should be monitored prior to the start of therapy, every 2 weeks during the first month of treatment, and then once a month thereafter.20,31 Although no events have occurred in humans, early preclinical studies of capmatinib revealed the potential for photosensitivity reactions.31 Therefore, it is recommended that patients undergoing therapy with capmatinib use precautionary measures against ultraviolet radiation such as sunscreen or protective clothing during treatment.31 A commonly reported adverse reaction of all MET inhibitors is generalized and peripheral edema.20,22,30,31 Studies of all agents have reported varying degrees of edema, as high as 52% and 70% with capmatinib and tepotinib respectively.20,22,30,31 Other frequently reported adverse reactions of MET inhibitors include fatigue, nausea, vomiting, diarrhea, musculoskeletal pain, decreased appetite, and pyrexia (TABLE 3).20,22,30,31
Research Progress
Research into treatment algorithms for MET-aberrated NSCLC has advanced greatly in recent years and continues to yield new discoveries and results. The approvals of capmatinib and tepotinib provide patients with further options in MET-mutated metastatic NSCLC and offer value in the METex14 setting.13,19 Although both capmatinib and tepotinib have thus far only displayed salient efficacy in METex14 mutations, both agents have presented suspected utility in the MET-amplified setting and will be further evaluated in future studies.13,19 Data from early clinical studies of savolitinib + osimertinib have demonstrated progress in the amelioration of MET-driven resistance to EGFR TKIs and will continue to be studied in this setting.22 Additionally, results from the SAVANNAH study will validate the efficacy of savolitinib + osimertinib after pretreatment with only osimertinib, potentially exposing patients to fewer cytotoxic regimens and allowing for continuity of osimertinib therapy.22 Confirmatory results of glumetinib are pending, although early data show promise due to its potent antitumor and antiproliferative activity.30 Further data will elicit glumetinib’s clinical utility and capacity in the MET-mutated setting.
Conclusion
MET-selective TKIs offer another specific treatment in the paradigm of metastatic NSCLC. The approval and development of contemporary MET-directed agents represent the everchanging, exciting treatment developments for NSCLC. Providers and patients will look forward to future developments in MET-dysregulation and other novel therapies.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. PDQ Adult Treatment Editorial Board. PDQ non-small cell lung cancer treatment. Bethesda, MD: National Cancer Institute. Updated March 11, 2021. www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq. Accessed June 2, 2021.
2. Sholl LM. The molecular pathology of lung cancer. Surg Pathol Clin. 2016;9(3):353-378.
3. Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in lung cancer: will expectations finally be MET? J Thorac Oncol. 2017;12(1):15-26.
4. Salgia R. MET in lung cancer: Biomarker selection based on scientific rationale. Mol Cancer Ther. 2017;16(4):555-565.
5. Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048-3056.
6. Pruis MA, Geurts-Giele WRR, von der Thusen TJH, et al. Highly accurate DNA-based detection and treatment results of MET exon 14 skipping mutations in lung cancer. Lung Cancer. 2020;140:46-54.
7. Pasquini G, Giaccone G. C-MET inhibitors for advanced non-small cell lung cancer. Expert Opin Investig Drugs. 2018;27(4):363-375.
8. Reungwetwattana T, Liang Y, Zhu V, Ou SI. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: the why, the how, the who, the unknown, and the inevitable. Lung Cancer. 2017;103:27-37.
9. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298-2308.
10. Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67(9):4408-4417.
11. Tanizaki J, Okamoto I, Okamoto K, et al. MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol. 2011;6(10):1624-1631.
12. Landi L, Chiari R, Tiseo M, et al. Crizotinib in MET-Deregulated or ROS1-Rearranged Pretreated Non-Small Cell Lung Cancer (METROS): a phase II, prospective, multicenter, two-arms trial. Clin Cancer Res. 2019;25(24):7312-7319.
13. Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944-957.
14. ClinicalTrials.gov. Safety and efficacy of capmatinib (INC280) plus pembrolizumab vs. pembrolizumab alone in NSCLC with PD-L1 ³50%. Identifier: NCT04139317. Updated March 11, 2021. https://clinicaltrials.gov/ct2/show/NCT04139317?term=capmatinib&draw=2&rank=3. Accessed April 12, 2021.
15. ClinicalTrials.gov. Study evaluating efficacy and safety of capmatinib in combination with osimertinib in adult subjects with non-small cell lung cancers as second line therapy (GEOMETRY-E). Identifier: NCT04816214. Updated March 25, 2021.
https://clinicaltrials.gov/ct2/show/NCT04816214?term=capmatinib&draw=2&rank=6. Accessed April 12, 2021.
16. ClinicalTrials.gov. Study of capmatinib and spartalizumab/placebo in advanced NSCLC patients with MET exon 14 skipping mutations. Identifier: NCT04323436. Updated March 11, 2021. https://clinicaltrials.gov/ct2/show/NCT04323436?term=capmatinib&draw=2&rank=7. Accessed April 12, 2021.
17. Study of Safety and Efficacy of EGFR-TKI EGF816 in Combination With cMET Inhibitor INC280 in Non-small Cell Lung Cancer Patients with EGFR Mutation. NCT02335944. Updated October14, 2021. https://clinicaltrials.gov/ct2/show/NCT02335944?term=capmatinib&draw=3&rank=28. Accessed April 12, 2021.
18. Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET Exon 14 skipping mutations. N Engl J Med. 2020;383(10):931-943.
19. Clinical Trials.gov. A study of tepotinib plus osimertinib in osimertinib relapsed MET-Amplified NSCLC (INSIGHT 2). Identifier: NCT03940703. Updated April 9, 2021.
https://clinicaltrials.gov/ct2/show/NCT03940703?term=tepotinib&draw=2&rank=3. Accessed April 12, 2021.
20. Tepmetko (tepotinib) package insert. Rockland, MA; EMD Serono, Inc.; Revised February 28, 2021.
21. Jones RDO, Grondine M, Borodovsky A, et al. A pharmacokinetic-pharmacodynamic model for the MET tyrosine kinase inhibitor, savolitinib, to explore target inhibition requirements for anti-tumour activity. Br J Pharmacol. 2021;178(3):600-613.
22. Sequist LV, Han JY, Ahn MJ, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21(3):373-386.
23. Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31(4):507-516.
24. Gan HK, Millward M, Hua Y, et al. First-in-human phase l study of the selective MET inhibitor, savolitinib, in patients with advanced solid tumors: safety, pharmacokinetics, and antitumor activity. Clin Cancer Res. 2019;25(16):4924-4932.
25. Lu S, Fang J, Li X, et al. Phase II study of savolitinib in patients (pts) with pulmonary sarcomatoid carcinoma (PSC) and other types of non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping mutations (METex14). J Clin Oncol. 2020;38(15):9519-9519.
26. Yu Y, Zhang Q, Zhang J, Lu S. Prevalence of MET exon 14 skipping mutation in pulmonary sarcomatoid carcinoma patients without common targetable mutations: a single-institute study. J Cancer Res Ther. 2019;15(4):909-913.
27. Liu X, Jia Y, Stoopler M B, et al. (2016). Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol. 2016;34(8):794-802.
28. Li X, Wu D, Liu H, Chen J. Pulmonary sarcomatoid carcinoma: progress, treatment and expectations. Ther Adv Med Oncol. 2020;12:1758835920950207.
29. Ai J, Chen Y, Peng X, et al. Preclinical evaluation of SCC244 (glumetinib), a novel, potent, and highly selective inhibitor of c-Met in MET-dependent cancer models. Mol Cancer Ther. 2018;17(4):751-762.
30. Chen HJ, Yang JJ, Yang X, et al. A phase I clinical trial to assess the safety, pharmacokinetics, and antitumor activity of glumetinib (SC244) in patients with advanced non-small cell lung cancers (NSCLCs). J Clin Oncol. 2020;38(15 suppl).
31. Tabrecta (capmatinib) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; revised May 31, 2020.
To comment on this article, contact rdavidson@uspharmacist.com.






