US Pharm. 2021;46(4):HS2-HS9.
ABSTRACT: Cytomegalovirus (CMV) continues to be a major complication in allogeneic hematopoietic cell transplant (aHCT) recipients. Either of two approaches may be used to mitigate the development of CMV after aHCT: preemptive initiation of antiviral treatment upon evidence of viral replication or a primary prophylaxis strategy. Both methods are equally effective, and the strategy employed is based on the patient’s specific risks for CMV disease. Given the drugs presently available, both preemptive and prophylactic strategies have shortcomings in terms of toxicity, drug interactions, or costs. The pharmacist has an important role in the selection, dosing, monitoring, and management of CMV therapies in aHCT recipients.
Cytomegalovirus (CMV) continues to be one of the most significant complications to arise after allogeneic hematopoietic stem cell transplant (aHCT).1 CMV is a betaherpesvirus (a single, large, enveloped, linear, double-stranded DNA virus that is a member of the Herpesviridae family). Primary infection or reactivation of latent CMV after aHCT contributes to significant morbidity and mortality.2 Thanks to advances in the prevention and treatment of CMV in aHCT recipients, the mortality rate has decreased by 20% to 25% since the 1990s.3 CMV can cause a host of direct and indirect complications in almost any organ system, contribute to multiorgan failure, and result in immunosuppression, which predisposes the patient to concurrent infections.1 Reactivation of CMV after aHCT has prognostic significance and continues to be a major contributor to nonrelapse mortality. This article will discuss the pathogenesis, diagnosis, risk factors, prevention, and treatment of CMV in aHCT recipients.
Pathogenesis
CMV is ubiquitous in the environment, and it can also be transmitted via an infected host’s bodily fluids (urine, saliva, breast milk, tears), blood transfusions, and vertical transmission.3 Primary infection with CMV in an immunocompetent host can either be asymptomatic or manifest in low-grade, self-resolving, flulike symptoms. As with other herpesviruses, once the host is exposed to CMV, the virus establishes latency upon resolution of the acute illness. Thereafter, once the host is exposed to systemic immunosuppression or becomes immunocompromised secondary to an underlying condition, reactivation of latent CMV occurs. Reactivation can result in two distinct syndromes: CMV infection and CMV disease.2 CMV infection is described as virus isolation or detection of viral proteins in a bodily-fluid or tissue specimen without overt symptoms of disease. CMV disease is defined by evidence of disease via viral diagnostic strategies in addition to attributable signs and symptoms. Owing to the pervasive environmental presence of CMV, the seroprevalence of this infection in the adult population ranges from 40% to 100%.4
Risk Factors
The most important risk factor for CMV is the serologic status of the donor and the recipient. The highest risk of CMV infection occurs when the recipient is seropositive (R+) and the donor is seronegative (D–) because of the lack of donor-transferred CMV-specific antibodies that could protect the host during reactivation of latent CMV.2,3 The lowest risk is when both the donor and recipient are seronegative (R–/D–), but some risk remains given the ubiquitous presence of CMV in the environment. Patients with D+/R– or D+/R+ status have an intermediate risk of CMV reactivation, as approximately 30% of individuals develop primary infection.2 Other important risk factors for CMV include age greater than 20 years, acute or chronic graft-versus-host disease, graft failure, high-dose (HD) corticosteroids, intensity of immunosuppression, T cell–depleted graft, antithymocyte globulin or alemtuzumab, and mismatched or unrelated donors.1,3
Complications
The clinical sequelae of CMV infection in immunocompromised patients may be classified into direct and indirect effects. CMV infiltrates cells in a variety of sites, including the endothelium, epithelial tissue, hematopoietic cell lines, and smooth muscles.1 Therefore, with active replication of viral components in these tissues, CMV can manifest as multiorgan failure (e.g., pneumonitis, hepatitis, colitis, encephalitis, retinitis). Gastrointestinal (GI) complications are the most common manifestation, as CMV can infiltrate the entire GI tract; infiltration of the upper tract causes nausea and anorexia, and infiltration of the lower tract results in ulceration and diarrhea.1 CMV pneumonitis is the most serious complication, with a mortality rate of up to 50% in HCT recipients, although its incidence has been significantly reduced with the advent of appropriate prophylaxis.1,5 Owing to the direct effect on hematopoietic cells, CMV can cause profound cytopenias that indirectly predispose previously immunodeficient patients to further infections from bacterial and fungal pathogens.
Diagnostic Methods
Although serologic evaluation of the patient’s pretransplant CMV-specific antibodies is useful in determining the risk of CMV, this diagnostic measure cannot be used if reactivation is suspected. Polymerase chain reaction (PCR) is the most sensitive method for detecting actively replicating CMV in aHCT recipients.5 The PCR test detects the presence of replicating CMV DNA in whole blood or plasma. PCR is often performed at specific intervals post aHCT to determine changes in viral load, and any increases above the test’s threshold and the institution-specific threshold would prompt CMV treatment. Another test that may be performed identifies CMV-specific pp65 antigen in the peripheral blood lymphocytes. This test is not as sensitive or specific as PCR and may not be useful in patients with low neutrophil counts.5 Resistance testing may be performed if CMV strains do not respond to conventional first-line therapies.2
Treatment Strategies
Without adequate treatment, approximately 80% of seropositive CMV patients experience reactivation after aHCT.1 The past decade saw major advances in the prevention and treatment of CMV that have significantly reduced the prevalence of CMV reactivation. Two strategies exist to combat CMV after aHCT: prophylaxis and preemptive therapy. Primary prophylaxis is the routine administration of antiviral agents (letermovir, HD acyclovir, valacyclovir, and—rarely—ganciclovir or valganciclovir) in patients at risk for infection. In preemptive therapy, PCR is performed periodically, and if viral copies above the institution-specific threshold are detected prior to the emergence of symptoms, antiviral treatment is initiated. This treatment, which comprises an induction phase followed by maintenance, may be discontinued once CMV is undetectable on PCR or another test used to identify CMV. Agents used for preemptive therapy include valganciclovir, ganciclovir, foscarnet, and cidofovir.2
Each modality comes with risks and benefits. Primary prophylaxis subjects low-risk patients to drug-related toxicities, unnecessary costs, and drug-drug interactions. Preemptive therapy relies on the patient’s ability to adhere to routine monitoring and early detection of viral copies in order to minimize complications of CMV disease. The decision regarding which strategy to use is based on patient-specific risk determined by the serologic status of the donor and recipient. In the following sections, the agents used for preventive therapy and preemptive therapy will be reviewed. See TABLE 1 for more information.
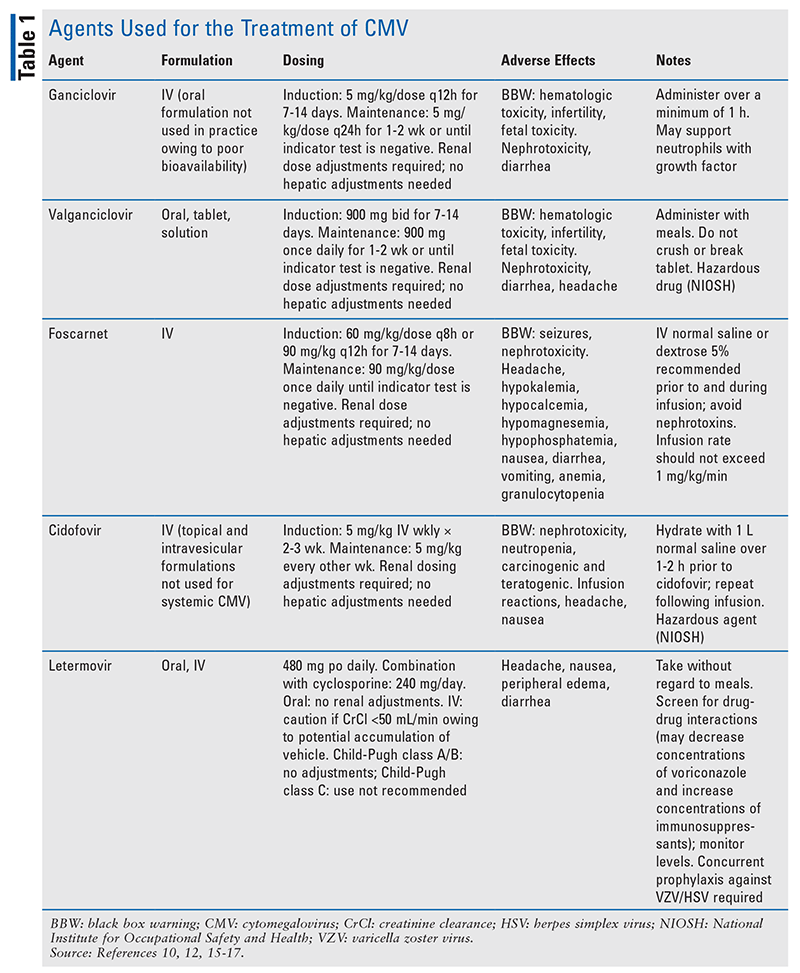
Acyclovir and Valacyclovir: Acyclovir is a guanosine analogue that inhibits DNA synthesis and viral replication by competing with deoxyguanosine triphosphate for viral DNA polymerase and incorporation into viral DNA.6 HD acyclovir has been shown to reduce the risk of CMV infection compared with low-dose acyclovir.7 Valacyclovir, a valine ester prodrug of acyclovir with improved bioavailability, has been associated with reduced rates of viremia compared with HD acyclovir in CMV R+ or D+ aHCT recipients.8,9 However, studies with HD acyclovir and valacyclovir illustrate that despite a reduction in CMV viremia, breakthrough CMV disease and its complications still occur, and resistance to these treatments is common.2 In practice, prophylaxis with acyclovir or valacyclovir is typically reserved for patients who are R–/D– (i.e., lowest risk of CMV). A benefit of prophylaxis with these therapies is their broad-spectrum antiviral activity, as they provide coverage against herpes simplex virus (HSV), varicella zoster virus (VZV), and other viruses that commonly occur after aHCT. Acyclovir and valacyclovir are not routinely used for preemptive therapy or primary prophylaxis in intermediate-risk or high-risk patients.
Ganciclovir and Valganciclovir: Ganciclovir is an analogue of 2-deoxyguanosine, a competitive substrate for UL54-encoded CMV DNA polymerase.10 Ganciclovir is triphosphorylated intracellularly by kinases and incorporated into DNA during viral replication, resulting in premature termination of DNA synthesis.10 IV ganciclovir has significantly greater antiviral activity against CMV compared with acyclovir and valacyclovir, and it has been shown to reduce the burden of both CMV infection and CMV disease.11 However, the routine use of ganciclovir, particularly in the prophylactic setting, has been limited owing to its black box warning (BBW) for nephrotoxicity and myelosuppression. Despite the substantial reduction in risk of CMV disease noted in clinical trials of ganciclovir, a survival benefit was not seen; this was due to the emergence of secondary bacterial and fungal infections attributed to ganciclovir’s myelosuppressive properties.1,2,11 Additionally, ganciclovir is associated with fertility impairment in both men and women, warranting appropriate contraception and informed discussion prior to treatment initiation.
Valganciclovir is the oral prodrug of ganciclovir, and pharmacokinetics demonstrates that equivalent and potentially higher drug exposure can be achieved compared with IV ganciclovir.12-14 Efficacy and safety are similar between these two therapies; however, because of CMV’s severity, oral valganciclovir is considered only in patients without invasive disease who can tolerate oral medications. In practice, valganciclovir may be used for maintenance therapy after induction with ganciclovir in order to facilitate outpatient treatment.
Foscarnet: This pyrophosphate analogue acts by binding to the pyrophosphate binding site encoded on viral polymerases, and it serves as a noncompetitive inhibitor of these enzymes.15 Foscarnet, an IV therapy, has been shown to be as effective as ganciclovir for preemptive treatment; however, it has serious toxicities and is typically reserved for patients who fail ganciclovir therapy because of resistance or dose-limiting neutropenia.2 Compared with ganciclovir, foscarnet has a lower incidence of myelosuppression, which is of significant benefit in patients undergoing aHCT because it facilitates engraftment. However, foscarnet carries BBWs for renal impairment and for seizures secondary to electrolyte disturbances.15 Nephrotoxicity, occurring in 30% to 50% of patients, is caused by the deposition of foscarnet crystals in the glomerular capillary lumen.15 Therefore, the patient should be hydrated prior to each infusion and monitored routinely for serum creatinine and electrolytes for the duration of use. In addition, foscarnet has highly complex renal dosing adjustments. The dosage and dosing frequency are based on creatinine clearance divided by body weight and should be adjusted based on renal-function changes during treatment.15
Cidofovir: This agent is an acyclic cytidine deoxynucleotide phosphate analogue that acts as a competitive inhibitor of viral DNA polymerase.16 Cidofovir is reserved as a third-line option for preemptive therapy or treatment of CMV not responding to ganciclovir or foscarnet secondary to its serious toxicity profile.2 Cidofovir has a BBW for nephrotoxicity based on cases of renal failure requiring dialysis and resulting in fatalities.16 Serum creatinine should be monitored for 48 hours prior to each dose of cidofovir, and dose modifications should be made promptly if there are changes in renal function. To minimize the likelihood of nephrotoxicity, probenecid administered prior to, during, and after cidofovir infusion can decrease renal tubular accumulation of cidofovir, prolong its half-life, and reduce the dose required.16 The patient should receive IV-fluid hydration prior to each cidofovir infusion. If tolerated and the patient is not at risk for complications of volume overload, a second bag of fluids may be administered during or after infusion.16 Cidofovir also has a BBW for neutropenia. This agent is available only as an IV formulation, making long-term treatment difficult. Compared with alternative therapies that are dosed daily, cidofovir’s long intracellular half-life (>24 hours) allows for less-frequent administration (every 1-2 weeks).16
Letermovir: This drug, the newest agent in the armamentarium for CMV prophylaxis in aHCT recipients, has a unique mechanism of action distinct from that of other CMV agents. Letermovir inhibits the viral terminase complex, a component involved in DNA cleavage and packaging that results in accumulation of immature viral DNA.17 The viral terminase complex exists only in CMV; because it is not found in human cells, overall toxicity is lessened and the risk of cross-resistance with other therapies is reduced. FDA approval of letermovir use in adult aHCT recipients seropositive for CMV was based on a multicenter phase III trial that demonstrated a reduced incidence of clinically significant CMV disease and reduced all-cause mortality at 48 weeks after aHCT compared with placebo.18 Letermovir has not been studied in a randomized, controlled trial for preemptive therapy or treatment of CMV, and it should be used only for primary prophylaxis.
Letermovir is available in both IV and oral formulations; it is initiated on day 5 after aHCT and continued until at least day 100.17 Overall, prophylaxis with letermovir is very well tolerated, but some patients may experience nausea and peripheral edema.18 Letermovir does not have the myelosuppressive effects and renal toxicity that restrict the use of other agents; however, unlike other therapies used for CMV, it has drug-drug interactions that are clinically significant in the aHCT population. Letermovir is a substrate of CYP3A4, CYP2D6, UGT1A1, UGT1A3, and the transporters P-glycoprotein (Pgp) and organic anion transporter (OAT) P1B1/3. A 50% dose reduction of letermovir is required when combined with cyclosporine.17 Because letermovir is a moderate CYP3A inhibitor and inhibits CYP2C8, Pgp, OAT3, and OATP1B1/3, it increases cyclosporine, tacrolimus, and sirolimus exposure, requiring more frequent monitoring of serum drug concentrations. Letermovir is also an inducer of CYP2C9 and CYP2C19, which decreases exposure to voriconazole, necessitating vigilant monitoring of voriconazole levels and dose modifications. Various other drug interactions are described in the product’s package insert and in TABLE 1. Another important caveat is that letermovir is active only against CMV; it does not act against other herpesviruses, including HSV and VZV.17
The Pharmacist’s Role
The pharmacist can guide the multidisciplinary team on the appropriate treatment strategy—either primary prophylaxis or the preemptive approach—based on the aHCT patient’s risk stratification determined via serologic testing for CMV antibodies. After risk determination, the pharmacist can support the patient throughout therapy in order to reduce the toxicity of older treatments or the financial burden of new therapies. Pharmacists should be antiviral stewards and prevent overlaps in antiviral coverage. Ganciclovir, valganciclovir, cidofovir, and foscarnet have coverage against other viruses relevant in the posttransplant setting (e.g., VZV, HSV). Therefore, if treatment with one of these agents is initiated, prophylaxis with acyclovir or valacyclovir may be stopped and then reinitiated once treatment is discontinued. However, it is prudent to continue prophylaxis with acyclovir or valacyclovir when letermovir is initiated for prophylaxis in order to mitigate the risk of other viral infections, as this agent covers only CMV.
Letermovir, albeit the most reasonable agent for prophylaxis owing to its efficacy and lack of toxicities, comes with a significant financial burden for the patient. Pharmacists should screen and anticipate patients who are candidates for letermovir prophylaxis and assess insurance approval prior to aHCT. Early assessment of the drug’s cost enables the pharmacist to work with the patient and the multidisciplinary team to devise a cost-effective and appropriate plan. A coupon card for copayment assistance is available for patients with private insurance.
For patients stratified to a preemptive strategy, the pharmacist can help improve the toxicity of traditional anti-CMV therapies; screen the patient for other nephrotoxic therapies; and discontinue, taper, or find safe alternative treatments in order to mitigate this serious adverse effect. For patients with neutropenia on therapy, growth-factor support may be considered to help reduce the risk of secondary infections. The pharmacist has an important role in the selection, dosing, monitoring, and management of CMV therapies in aHCT recipients.
REFERENCES
1. Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711-5719.
2. Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(8):e260-e272.
3. Zaia JA. Prevention and management of CMV-related problems after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29(8):633-638.
4. Staras SAS, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143-1151.
5. Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010;24(2):319-337.
6. Acyclovir package insert. East Windsor, NJ: AuroMedics Pharma LLC; January 2021.
7. Prentice HG, Gluckman E, Powles RL, et al. Impact of long-term acyclovir on cytomegalovirus infection and survival after allogeneic bone marrow transplantation. European Acyclovir for CMV Prophylaxis Study Group. Lancet. 1994;343(8900):749-753.
8. Valtrex (valacyclovir) package insert. Research Triangle Park, NC: GlaxoSmithKline; December 2020.
9. Ljungman P, de La Camara R, Milpied N, et al. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood. 2002;99(8):3050-3056.
10. Ganciclovir package insert. Schaumburg, IL: Sagent Pharmaceuticals; December 2020.
11. Einsele H, Ljungman P, Boeckh M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. 2020;135(19):1619-1629.
12. Valcyte (valganciclovir) package insert. South San Francisco, CA: Genentech USA, Inc; January 2021.
13. Einsele H, Reusser P, Bornhäuser M, et al. Oral valganciclovir leads to higher exposure to ganciclovir than intravenous ganciclovir in patients following allogeneic stem cell transplantation. Blood. 2006;107(7):3002-3008.
14. van der Heiden PLJ, Kalpoe JS, Barge RM, et al. Oral valganciclovir as pre-emptive therapy has similar efficacy on cytomegalovirus DNA load reduction as intravenous ganciclovir in allogeneic stem cell transplantation recipients. Bone Marrow Transplant. 2006;37(7):693-698.
15. Foscavir (foscarnet) package insert. Lake Forest, IL: Hospira, Inc; December 2020.
16. Vistide (cidofovir) package insert. Foster City, CA: Gilead Sciences, Inc; January 2021.
17. Prevymis (letermovir) tablets and injection package insert. Whitehouse Station, NJ: Merck & Co, Inc; January 2021.
18. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433-2444.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






