US Pharm. 2022;47(6):33-36.
ABSTRACT: As frontline healthcare providers, pharmacists are well-poised to provide patients with pertinent information about generic drugs as well as ease patient concerns about generic drugs versus brand-name drugs. Pharmacists can be influential in counseling patients about generic drugs versus brand-name drugs and in answering questions about active versus inactive ingredients to ensure that patients have a thorough understanding about generic drugs. In turn, this may improve patient outcomes through cost savings and patient adherence.
The FDA indicates that in the United States, nine out of ten prescriptions filled are for generic drugs, and in general, generic drugs cost an estimated 85% less than brand-name drugs.1,2 According to the FDA, the agency approves more than 800 drug applications for generic drugs annually.2 The FDA also indicates that the growing number of generic drugs entering the market continue to generate competition in the marketplace as well as assist in making many drugs more affordable, which may expand patient access to drugs that they may have not otherwise been able to afford.1 Findings from a recent global generic drugs market report indicated that in 2021 the generic-drug market reached a value of $320 billion and that the market is projected to reach $482.5 billion by 2027.3
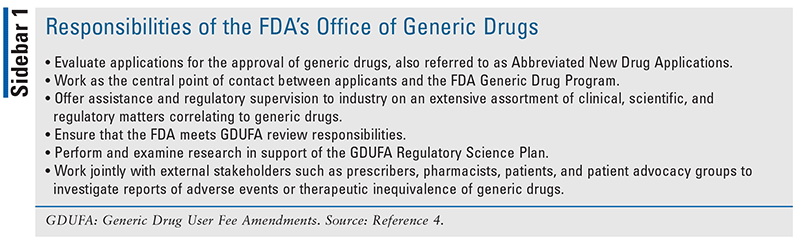
The FDA’s Office of Generic Drugs (OGD), which is a component of the Center for Drug Evaluation and Research, is responsible for implementing the generic drug regulatory process and ensuring the safety and efficacy of generic drugs (see SIDEBAR 1).4 The OGD ensures that the manufacturing, packaging, and testing sites for generic drugs meet and adhere to the identical quality standards as those required for brand-name drugs.4 Additionally, they ensure that generic drugs undergo a meticulous review and approval process.1,4
In an effort to expand patient-education initiatives about generic drugs and to ensure that consumers understand that generic drugs have the same safety, efficacy, and quality standards as their brand-name counterparts, the FDA launched a campaign in September 2017 to increase cognizance about the value of generic drugs.6 Moreover, the FDA notes that pharmacists can be influential in reiterating these messages to their patients. The FDA encourages patients and their prescribers to converse with one another about exploring generic alternatives to brand-name medications.6
In 2017, the FDA announced the Drug Competition Action Plan (DCAP) to further inspire vigorous and judicious market competition for generic drugs and aid in conveying better efficiency and clarity to the generic-drug review process without forfeiting the scientific thoroughness fundamental to the FDA generic-drug program.7 Through the DCAP, the FDA is assisting in eradicating barriers to generic-drug development and market entry in an effort to encourage competition so that consumers can obtain the medications they need at reasonable prices.7
Common Myths and Misconceptions About Generic Drugs
Both brand-name and generic drugs are FDA approved and tested for safety and efficacy. The FDA ensures that the manufacturers of generic drugs meet the same batch-to-batch requirements for strength, purity, and quality as the original brand-name manufacturer and adhere to the same strict “Good Manufacturing Practices” rules.8-10 There are many myths and misconceptions about generic drugs, and pharmacists can dispel them by providing patients with the facts about generic drugs, as illustrated in TABLE 1.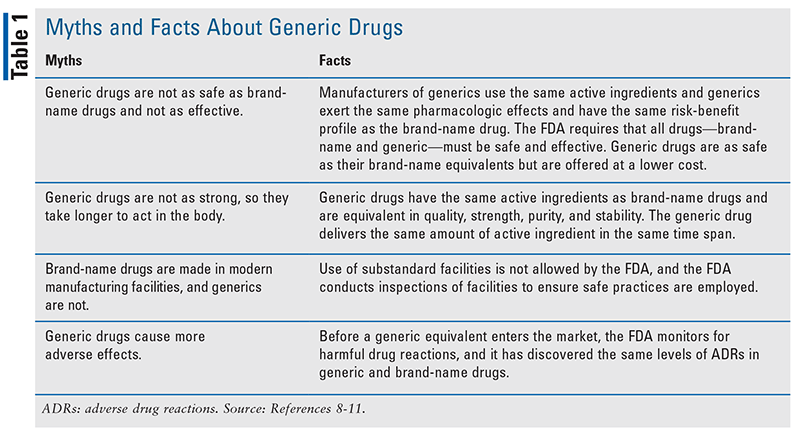
Brand-Name Drugs Versus Generic Drugs
The FDA necessitates that the generic drugs have the same active ingredient, strength, dosage form, and route of administration (ROA) as the brand-name drug. In doing so, the manufacturer of the generic drug must prove its formulation is the same (bioequivalent) as the brand-name drug. The FDA requires that all manufacturing, packaging, and testing sites pass the identical quality standards as those of brand-name drugs. Additionally, many generic drugs are made in the same manufacturing plants as brand-name drugs.9,10
When educating patients about generics, pharmacists can also relay the following information to address patient concerns9,10:
• The FDA considers several factors when approving a generic formulation of a medication.
• Generic drugs undergo a thorough review process to acquire FDA approval.
• The FDA ensures a generic medication provides the same clinical benefit and is as safe and effective as the brand-name medicine.
• Generic medicines tend to cost less than their brand-name counterparts because they do not have to repeat animal and clinical (human) studies that were required of the brand-name medicines to determine safety and effectiveness.
• Once a generic medication is available, the FDA continues to screen its safety, efficacy, and quality.
• The FDA monitors their Adverse Event Reporting System and reviews MedWatch reports to explore concerns related to generic-drug product quality and therapeutic inequivalence.
Generic Drug Findings
Numerous studies have been conducted in several countries to evaluate consumer attitude and behavior when purchasing generic drugs. Publications reveal that perceptions regarding purchasing these generic drugs can be influenced by the perceived quality, product characteristics, past experiences, and healthcare provider’s recommendations.12
A national survey evaluated “generic skepticism,” defined as not thinking generic drugs were as safe, effective, had the same AEs, and contained the same active ingredients as brand-name drugs.13 Researchers also evaluated how frequently patients requested that their physicians prescribe a brand-name over a generic drug in the past year. Findings revealed that of the 1,442 patients, 933 responded (65% response rate) and 753 took the full survey.13 The majority (83%) agreed that physicians should prescribe generic drugs when available, and 54% stated they had not requested that their physicians prescribe a brand-name drug over a generic drug in the past year. Most respondents considered generic drugs to be as effective (87%) and safe (88%) as their brand-name counterparts and that generic drugs have the same AEs (80%) and active ingredients (84%). The authors concluded that there is a considerable trend towards more patients having positive opinions about generic drugs, but persistent negative opinions will have to be addressed to witness the continued cost savings and improved patient outcomes from generic drugs.13
Another study aimed to identify the contributing factors of generic-drug substitution across classes. The authors indicated that generic substitution rates for most drug classes exceeded 90%, although some were much lower, including thyroid hormones (64%), androgens (74%), estrogens (71%), and hydantoin-type anticonvulsants (72%).14 In five of the eight studies, the authors found that patients using a mail-order pharmacy had considerably less generic substitution than patients using retail pharmacies; two additional classes disclosed no relationship between pharmacy type and generic use.14 They also noted that older patients are less prone to believe that generic drugs are as safe as branded drugs, and women are more prone than men to believe that generics are a better value. Individuals who were taking multiple medications were more likely to use generics as well.14
In a comparison study of U.S. health insurance claims, researchers found that branded and generic drugs had comparable medical results for chronic conditions that included hypertension, diabetes, osteoporosis, and psychiatric conditions such as anxiety and depression.15 The authors noted their findings demonstrate that implementing educational interventions targeted at expanding both prescriber and patient confidence in generic drugs may augment use of generics for treatment of chronic medical conditions.15
Generic Drug Publications
In a recent publication, researchers revealed that there are three prime categories that tend to influence an individual’s attitude toward—and intention to purchase—generic drugs: 1) consumer attitude and behavior, 2) patients’ and health professionals’ perceptions about generics, and 3) risks related with generic drugs.16 The authors of the study also noted that there is a need for the pharmaceutical industry, public health policymakers, and healthcare professionals to gain a better comprehension about patient attitudes and behavior toward generic medicines, which in turn could also augment the use and acceptance of generic drugs.16In a recent publication, authors attempted to ascertain pharmacists’ preferences of generic versus brand OTC medication for their personal use with the various ailments available to self-treatment. The researchers conducted a prospective, cross-sectional study involving over 500 pharmacists. Overall, the study findings indicated that pharmacists preferred generic OTC drugs to brand OTC drugs (62% vs. 5%, respectively).17 The authors indicated that pharmacists were prone to use generic formulations of OTC drugs for self-management of health symptoms (average = 7.32 ± 2.88). In addition, pharmacists chose generic OTC drugs over brand drugs regardless of health symptoms (P <.001). The authors concluded that pharmacists had a considerable preference for choosing generic drugs versus brand drugs across various symptoms such as aches, allergies, cough, acid reflux, constipation, insomnia, daytime/nighttime cold and flu, and pain. Overall, the study provided proof that pharmacists prefer generic medication over brand medication for their personal use as self-treatment.17
Recent News From the FDA
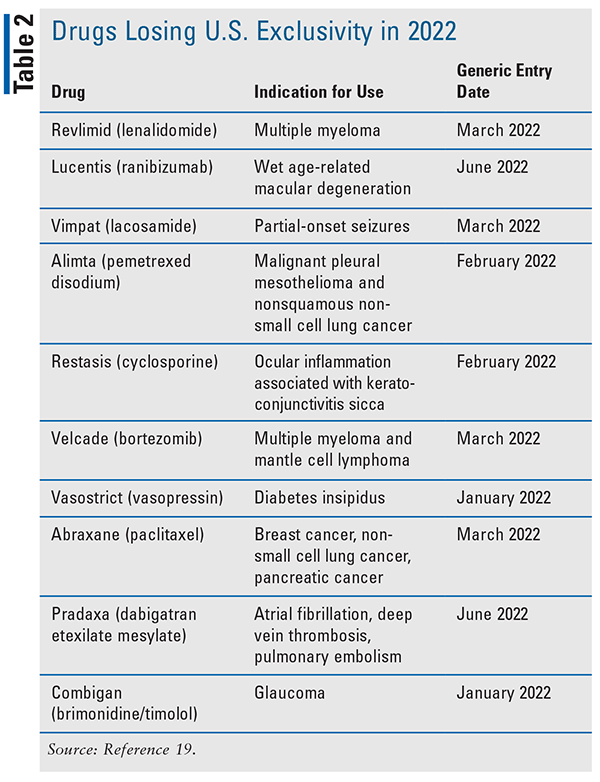
In a press release on January 26, 2022, the FDA published a series of guidances focused on generic-drug application submissions, labeling, and review.18 The guidances are in accordance with, and in support of, the FDA’s DCAP.18Pharmacists can also keep patients up to date with brand-name drugs that lose U.S. exclusivity this year, as indicated in TABLE 2.
The Role of the Pharmacist
Literature clearly illustrates the valuable role of the pharmacist in patient care, and their interventions can have a measurable effect on overall clinical outcomes. Pharmacists can be influential in counseling patients about generic drugs versus brand-name drugs and in answering questions about active versus inactive ingredients to ensure that patients have a thorough understanding about generic drugs. In turn, this may improve patient outcomes through cost savings and patient adherence. Engaging patients in conversation about generic drugs may help in alleviating their concerns about the safety and effectiveness of generic drugs, promote confidence in their use, and provide them with cost-saving options.During counseling, pharmacists can remind patients that the FDA ensures generic drugs contain the same active/key ingredient, have the same strength/safety/efficacy, use the same dosage form, and have the same ROA as their brand name product. Pharmacists can also inform patients that since generic drugs are marketed by various manufacturers, generic drugs may have slight differences from the brand (e.g., different inactive ingredients, or variations in the color, shape, and size of their brand name counterparts) but will have the same pharmacologic effects and clinical benefits.
Valuable patient education resources about generic drugs can be found at:
Generic Drugs Facts: www.fda.gov/drugs/generic-drugs/generic-drug-facts.
Generic Drug Patient Education: www.fda.gov/drugs/generic-drugs/patient-education.
Generic Drug Questions & Answers: www.fda.gov/drugs/questions-answers/generic-drugs-questions-answers.
FDA Approval Process for Generic Drugs: www.fda.gov/drugs/generic-drugs/what-approval-process-generic-drugs.
REFERENCES
1. FDA. Generic drugs. February 5, 2021. www.fda.gov/drugs/buying-using-medicine-safely/generic-drugs. Accessed March 29, 2022.
2. FDA. Prescriber stakeholder toolkit–sample blog post/newsletter article. December 18, 2020. www.fda.gov/drugs/generic-drugs/prescriber-stakeholder-toolkit-sample-blog-postnewsletter-article. Accessed March 31, 2022.
3. Businesswire. Global generic drugs market report 2022: industry trends, share, size, growth, opportunity and forecast 2017-2027–ResearchAndMarkets.com. March 28, 2022. www.businesswire.com/news/home/20220328005612/en/. Accessed March 30, 2022.
4. FDA. Office of generic drugs. May 19, 2021. www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-generic-drugs. Accessed March 30, 2022.
5. Sacks CA, Van de Wiele VL, Fulchino LA, et al. Assessment of variation in state regulation of generic drug and interchangeable biologic substitutions. JAMA Intern Med. 2021;181(1):16-22.
6. Bonner L. FDA promotes talking about generics in new public education campaign. Pharmacy Today. 2017;23(11):19. www.pharmacytoday.org/article/S1042-0991(17)31675-4/fulltext.
7. FDA. FDA drug competition action plan. February 4, 2022. www.fda.gov/drugs/guidance-compliance-regulatory-information/fda-drug-competition-action-plan. Accessed March 30, 2022.
8. BlueCross BlueShield of New Mexico. How does a drug become a generic: facts vs. myths. February 25, 2022. https://connect.bcbsnm.com/health-and-wellness/b/weblog/posts/the-battle-brand-vs-generic-learn-facts. Accessed March 30, 2022.
9. FDA. Patient education. June 19, 2018. www.fda.gov/drugs/generic-drugs/patient-education. Accessed March 30, 2022.
10. FDA. Generic drug facts. November 1, 2021. www.fda.gov/drugs/generic-drugs/generic-drug-facts. Accessed March 30, 2022.
11. Johns Hopkins University. Generic drugs: myths vs. facts. October 13, 2017. https://hub.jhu.edu/at-work/2017/10/13/generic-drugs-myths-vs-facts/. Accessed March 31, 2022.
12. Ferreira VL, Pereira da Veiga CR, Kudlawicz-Franco C, et al. Generic drugs in times of economic crisis: are there changes in consumer purchase intention? J Retail Consum Serv. 2017;37:1-7.
13. Kesselheim AS, Gagne JJ, Franklin JM, et al. Variations in patients’ perceptions and use of generic drugs: results of a national survey. J Gen Intern Med. 2016;31(6):609-614.
14. Segal JB, Onasanya O, Daubresse M, et al. Determinants of generic drug substitution in the United States. Ther Innov Regul Sci. 2020;54(1):151-157.
15. Desai RJ, Sarpatwari A, Dejene S, et al. Comparative effectiveness of generic and brand-name medication use: a database study of US health insurance claims. PLoS Med. 2019;16(3):e1002763.
16. Arcaro R, Pereira da Veiga CR, Vieira da Silva W, Pereira da Veiga C. Attitude and purchase intention to generic drugs. Int J Environ Res Public Health. 2021;18(9):4579.
17. Patel M, Slack M, Cooley J, Bhattacharjee S. A cross-sectional survey of pharmacists to understand their personal preference of brand and generic over-the-counter medications used to treat common health conditions. J Pharm Policy Pract. 2016;9:17.
18. FDA. FDA issues series of guidances under Drug Competition Action Plan. January 26, 2022. www.fda.gov/drugs/drug-safety-and-availability/fda-issues-series-guidances-under-drug-competition-action-plan. Accessed March 30, 2022.
19. Sagonowsky E, Dunleavy K, Kansteiner F. Fierce Pharma. The top 10 drugs losing US exclusivity in 2022. March 8, 2022. www.fiercepharma.com/special-reports/top-10-drugs-losing-us-exclusivity-2022. Accessed March 30, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






