US Pharm. 2020;45(9):17-20.
ABSTRACT: Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States. Most HPV infections are asymptomatic and resolve spontaneously within 1 to 2 years. About 80% of people will get HPV in their lifetime. Some HPV infections that last beyond 12 months can increase risk of cancer and precancer. Gardasil9 is the only vaccine approved in the U.S. for prevention of HPV-associated cancers and precancerous lesions. Despite the availability of an effective vaccine, millions of Americans remain susceptible to HPV infection and its associated cancers. Pharmacists have an essential role in promoting public health through proper education of patients and improvement in immunization rates against this preventable infection.
Human papillomavirus (HPV) is a double-stranded DNA virus that occurs mostly in individuals between the ages of 15 and 25 years.1 There are over 100 types of HPV that can affect any location in the body, from the human genital tract to the oropharyngeal tract. HPV targets the basal keratinocytes after a wound allows human cells to be exposed to the virus. HPV types 6 and 11 are responsible for benign genital warts and are considered low risk for complications. HPV types 16 and 18 are classified as high risk and cause dysplastic lesions that account for 80% of all HPV-associated cancers and over a quarter of a million deaths annually.2,3 Most infections are asymptomatic and resolve within 12 months; however, carcinogenic HPV infections that last beyond 12 months may increase the risk of precancer or cancer.2
Nearly 80 million people are currently infected with HPV in the United States, and approximately 14 million people, including teenagers, become infected with HPV each year. Prevalence of genital infection with any HPV type was 42.5% among adults aged 18 to 59 years during 2013-2014.4 Prevalence of any high-risk genital and oral HPV infection was higher among non-Hispanic black adults and lower among non-Hispanic Asian adults.4 HPV infections and cervical precancers have decreased since the introduction of a vaccine, but immunization rates still fall below the target of 80% set by Healthy People 2020.5
Risk Factors
The most common risk factor for genital HPV infection is sexual activity. HPV can be transmitted even when an infected person is asymptomatic. Other risk factors are acquisition of new sex partners, having multiple sex partners, a history of sexually transmitted disease, and inconsistent condom use. Penetrating vaginal intercourse is not required for transmission of the HPV virus. Men who have sex with men are at higher risk for contracting anal HPV than are heterosexual men.6
HPV and Cancer
Persistent, oncogenic, high-risk HPV infection is a main risk factor for developing HPV-associated cancers and precancers. Nearly all cases of cervical cancer are related to HPV infection, with HPV type 16 accounting for about 50% of cases and HPV type 18 accounting for 20% of cases.1 Routine cervical screening should be started at age 21 and continue through age 65 years to prevent invasive cervical cancer. A Pap test, which involves collecting cervical cells to screen for changes or development of lesions that may lead to cervical cancer, is recommended every 3 years for women ages 21 to 65 years; women aged 30 to 65 years should also have an HPV test. Women who test negative for both the HPV and Pap tests can lengthen the screening interval to 5 years.7
Pap tests have played a large part in reducing cervical cancer incidence and mortality. A common misconception is that a Pap test can replace the HPV vaccine to protect against cervical cancer. Pap tests can only identify cervical precancers, and they fail to address other HPV-related anogenital or oropharyngeal cancers. While there are 12,000 new HPV-related cases of cervical cancer per year nationally, there are also 12,000 new cases of vaginal, vulvar, anal, and oropharyngeal cancers in women and 19,000 new cases of anal, penile, and oropharyngeal cancers in men.8
HPV types 16 and 18 cause almost 90% of anal cancers and precancerous anal lesions. Overall, women have a higher incidence of anal cancer than do men, but a lower incidence compared with men who have sex with men.2 HPV infection accounts for approximately 39% to 43% of cases of vulvar cancer, with HPV types 16 and 18 causing approximately 35% to 77% of HPV(+) vulvar cancers; 35% to 40% of penile cancers; and 70% to 80% of HPV(+) penile cancers.1 HPV infections also play a role in the development of oropharyngeal cancers, cancer found around the oropharynx and base of the tongue and tonsils.
ACIP Recommendations
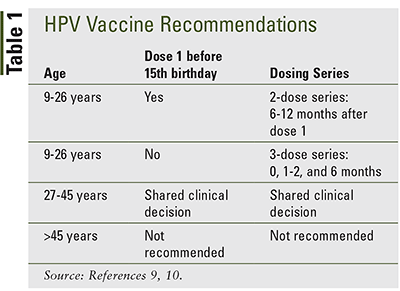
The Advisory Committee on Immunization Practices (ACIP) provides recommendations on the administration of routine vaccines, which includes the appropriate timing, dosage, and contraindications of each vaccine. The first dose of the HPV vaccine, 9vHPV (Gardasil9), is recommended to be started in children aged 11 to 12 years but can be given as early as age 9 years. The second dose of the vaccine is recommended 6 to 12 months after the first dose, for a total of 2 doses. Children who receive their first dose of the HPV vaccine after their 15th birthday or those who are immunocompromised require a three-shot series (at 0, 1-2, 6 months), given over the course of 6 months.9
Catch-up vaccinations through age 26 are recommended for both males and females who have not been adequately vaccinated. Adults older than age 26 years may receive the vaccine based on a shared clinical decision with a healthcare provider, weighing benefits and risks, due to the minimal public health benefit of the vaccine in this age group.10 A three-dose schedule is recommended for those between 27 and 45 years of age. The HPV vaccine is not licensed for use in those over age 45 years. Anyone who is pregnant, has had a life-threatening allergic reaction to any ingredients of the HPV vaccine, or has an allergy to yeast should not get the vaccine. Those who are breastfeeding can receive the HPV vaccine10 (TABLE 1).
Vaccines
In December 2014, there were three approved vaccines for HPV: Cervarix (2vHPV), Gardasil (4vHPV), and Gardasil9 (9vHPV), all of which are synthetically manufactured virus-like particles. Gardasil, the first available vaccine against HPV, was approved for use in the U.S. in 2006 to protect against four HPV types associated with cancers: 6, 11, 16, and 18.11 It is indicated for prevention of genital warts, precancerous or dysplastic lesions, and cervical cancer in females and males ages 9 to 26 years.12 Gardasil was then replaced by Gardasil9 in 2014; Gardasil9 was approved for use against the same four HPV types covered by Gardasil, plus five additional types: 31, 33, 45, 52, and 58.13 The 9-valent vaccine also contains more than twice the antigenic load for HPV16 and HPV18, the two most prevalent types associated with cervical cancer, proving noninferiority in antibody responses to Gardasil.13 It is indicated for prevention of genital warts, precancerous or dysplastic lesions, and cervical, vulvular, vaginal, and anal cancers in females 9 to 45 years old.14 Cervarix is equivalent in efficacy to Gardasil9 in preventing HPV infections and HPV-associated cancers.11 It is bivalent and targets HPV16 and HPV18, but it is no longer available in the U.S. due to its lower antigenic concentration than that of both Gardasil and Gardasil9. Gardasil9 is the only HPV vaccine currently available in the U.S.
Pervasive myths and misconceptions surrounding vaccines often serve as a barrier to optimal immunization rates. As a vaccine for a sexually transmitted infection, the HPV vaccine consistently lags behind other routine vaccines for adolescents. Many parents assume their preteen children are not sexually active, fearing that immunization may actually encourage sexual activity. However, research indicates that HPV vaccination in the recommended ages did not demonstrate any significant increase in sexual outcomes.8 HPV is not spread exclusively through sexual contact—although rare, it can also be spread by skin-to-skin contact.15
The HPV vaccine is most effective when the series is completed before any sexual activity begins, which is one of the main reasons the vaccine is recommended for boys and girls at an early age. If administered and completed by age 12 years, the vaccine is able to elicit a greater immune response and antibody amount to fight infection.8 Clinical trial data show that both males and females who received the HPV vaccine at age 11 or 12 years had the highest antibody titers—approximately twice the amount in individuals vaccinated at age 15 years or older.16
Safety
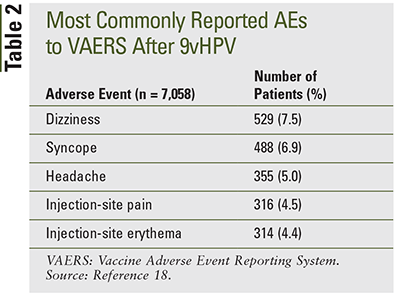
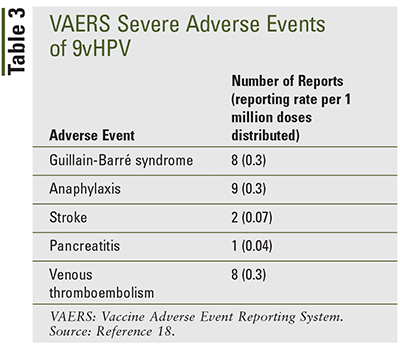
Each HPV vaccine underwent extensive safety testing before being FDA approved. Data collected from the Vaccine Adverse Event Reporting System (VAERS) from June 2006 to June 2017 showed that of more than 80 million doses of Gardasil administered, there were 36,142 reports of adverse events, with 96% of the adverse events being classified as nonserious. The most common adverse effects included dizziness, fatigue, injection-site reactions, and headache. Safety studies of Gardasil provided safety data that was relevant for Gardasil9.17 From December 2014 to December 2017, 28 million doses of Gardasil9 were distributed throughout the U.S. Postlicensure surveillance reports to VAERS showed that of 7,244 reports received after administration of Gardasil9, 97.4% were nonserious.18 Similar to its predecessor, the most common reported adverse events were dizziness, syncope, headache, and injection-site reactions (TABLE 2). Rare but severe adverse events of Gardasil9 included anaphylaxis, allergic reaction, appendicitis, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, injection-site reaction, pancreatitis, stroke, seizure, and venous thromboembolism17 (TABLE 3).
Additional data from Vaccine Safety Datalink (VSD) showed that of 838,991 doses administered from October 2015 to October 2017, there were eight cases of pancreatitis in men ages 18 to 26 who were exposed to the vaccine. A medical record review determined that seven of eight cases were either not incident or attributable to causes other than vaccination; thus, the relative risk (RR), although slightly elevated, was not statistically significant (RR = 4.7, P = 0.47).19 Thirty cases of acute appendicitis, the most frequent serious adverse event, were reported within 42 days of vaccination. However, further analysis failed to confirm causal association. VSD also found a postvaccination-related allergic reaction to be consistent with prelicensure clinical trials, in which serious allergic reactions were rare.19 After 2 years of weekly surveillance of the 9-valent HPV vaccine, no new safety concerns were identified. Meta-analysis of prospective controlled studies also found most adverse reactions to be transient.20
Parents may be hesitant to immunize their child based on the notion that vaccines pose a risk of infecting their child with the disease it is meant to prevent. Some may also believe that natural immunity is better than vaccine-acquired immunity. Not only have vaccines been extensively studied and proven safe but they are also continuously monitored for safety as long as they are being used. Vaccines are capable of causing adverse effects that may be confused with the disease itself. However, these adverse effects tend to be minor and can be treated with supportive care. Furthermore, the benefits of HPV vaccine far outweigh any potential risk of side effects. Within 10 years of Gardasil’s release, the CDC analyzed data reported by HPV-IMPACT and determined there had been an 86% decrease in HPV infections among those aged 14 to 19 years and a 71% decrease among those aged 20 to 24 years in the U.S.17 From 2008 to 2012, prevalence of HPV 16/18 in cervical intraepithelial neoplastic (CIN2+) lesions showed a statistically significant decrease from 53.6% to 28.4% among women who received at least one vaccine dose.21
Role of the Pharmacist
One pivotal role of a pharmacist is that of immunizer. With their extensive knowledge of the available vaccines, pharmacists serve as one of the most accessible healthcare professionals who can provide necessary information that will guide patients in making informed choices regarding vaccinations. Pharmacists can screen all patients, identify the need for the vaccine, and administer or refer the patient to another immunizer. Community pharmacists have the opportunity to ensure adherence by administering vaccines directly. Currently, the majority of states in the U.S. have pharmacists authorized to administer the HPV vaccine. They are able to address the concerns or fears of patients and parents by providing them with the facts. Pharmacists can discuss the benefits and risks of the HPV vaccine. Advising patients of their risk of infection and encouraging them to accept the immunizations can improve immunization rates. Pharmacists can consult with patients regarding the HPV vaccine face to face, by telephone, or by mail; awareness of the vaccine’s availability can also be increased with inserts attached to prescriptions.
Conclusion
HPV continues to be the most prevalent sexually transmitted infection with clinical consequences that can be prevented with vaccination. There are over 100 known types of HPV, some of which are responsible for development of certain cancers with high mortality rates. The current HPV vaccine available in the U.S. is Gardasil9, which can be administered in children as young as age 9 years. Pharmacists play an increasingly crucial role in improving vaccination rates in children by communicating with parents about the availability and importance of the HPV vaccine, as well as debunking any myths surrounding the vaccine.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Palefsky, J. Human papillomavirus infections: epidemiology and disease associations. In Hirsch M, ed. UpToDate. Waltham, MA: UpToDate; 2020. www.uptodate.com/contents/human-papillomavirus-infections-epidemiology-and-disease-associations. Accessed June 11, 2020.
2. Brown DR, Ermel A. Human papillomavirus infections. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine, 20th ed. https://accessmedicine.mhmedical.com/book.aspx?bookID=2129. New York, NY: McGraw-Hill. Accessed June 11, 2020.
3. McClung NM, Gargano JW, Park IU, et al. Estimated Number of Cases of High-Grade Cervical Lesions Diagnosed Among Women—United States, 2008 and 2016. MMWR Morb Mortal Wkly Rep. 2019;68(15):337-343.
4. CDC. Prevalence of HPV in adults aged 18-69; United States, 2011-2014. www.cdc.gov/nchs/products/databriefs/db280.htm. Accessed June 30, 2020.
5. Healthy People 2020. Immunization and infectious diseases. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed July 2, 2020. 6. Burgos R. Sexually transmitted infections. In: PharmacotherapyFirst: A Multimedia Learning Resource. American Pharmacists Association. https://pharmacylibrary.com/doi/10.21019/pharmacotherapyfirst.sti_overview. Accessed June 11, 2020.
7. CDC. HPV-associated cancers and precancers. www.cdc.gov/std/tg2015/hpv-cancer.htm. Accessed June 30, 2020.
8. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15(7-8):1628-1638.
9. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
10. CDC. HPV vaccine recommendations. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html#recommendations. Accessed June 27, 2020.
11. Harpe, D, DeMars LR. HPV vaccines—a review of the first decade. Gynecol Oncol. 2017;146(1):196-204.
12. Gardasil package insert. Whitehouse Station, NJ: Merck & Co; October 2016.
13. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Letters. 2020;471:88-102.
14. Gardasil9 package insert. Whitehouse Station, NJ: Merck & Co; June 2020.
15. CDC. About HPV. www.cdc.gov/hpv/parents/about-hpv.html. Accessed June 26, 2020.
16. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) [published correction appears in MMWR Recomm Rep. 2014;63(49):1182]. MMWR Recomm Rep. 2014;63(RR-05):1-30.
17. CDC. HPV vaccine safety and effectiveness data. www.cdc.gov/hpv/hcp/vaccine-safety-data.html. Accessed June 25, 2020.
18. Shimabukuro TT, Su JR, Marquez PL, et al. Safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019;144(6):e20191791.
19. Donahue JG, Kieke BA, Lewis EM, et al. Near real-time surveillance to assess the safety of the 9-Valent human papillomavirus vaccine. Pediatrics. 2019;144(6):e20191808.
20. Ogawa Y, Takei H, Ogawa R, Mihara K. Safety of human papillomavirus vaccines in healthy young women: a meta-analysis of 24 controlled studies. J Pharm Health Care Sci. 2017;3:18.
21. Hariri S, Bennett NM, Niccolai LM, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States—2008–2012. Vaccine. 2015;33(13):1608-1613.
To comment on this article, contact rdavidson@uspharmacist.com.





