US Pharm. 2012;37(6):39-42.
Approximately 15% of couples do not achieve pregnancy within one year of unprotected sexual intercourse.1-3 A male infertility factor is identified in about 50% of these cases and is solely responsible in 20% of couples.1,2 Male infertility has been attributed to a variety of causes including lifestyle factors, gonadotoxin exposure, hormonal dysfunction, chromosomal disorders, varicoceles, testicular failure, ejaculatory disorders, and obstruction. Evaluation of male infertility is important to identify a cause and provide treatment if the etiology is correctable. If a specific treatment is not available or the origin of the male factor infertility is not correctable, other options such as assisted reproductive techniques (ART) may exist. The pharmacist plays a vital role in identifying medications that contribute to male infertility, counseling the couple on medications used to treat infertility, and promoting healthy lifestyles that minimize infertility factors. The purpose of this article is to provide a broad overview of the etiology, evaluation, and treatment of male infertility.
Definitions and Epidemiology
Infertility is defined as the inability to achieve conception despite one year of regular, unprotected intercourse.4 In the United States, approximately 8 million couples are affected by infertility.5 For healthy young couples, the probability of achieving pregnancy within the first year of fertility-focused sexual activity is 84%.6 Despite advances in the diagnosis and treatment of infertility, the conception rate remains stable.6 A recent increase in demand for infertility services has been attributed to a greater awareness of treatment options, increased acceptance of infertility, and a trend toward delayed marriage and childbirth leading to more fertility issues.6
Male Reproductive Physiology
The testicles contain Leydig, Sertoli, and germ cells, which are responsible for the production of sperm and the synthesis of testosterone. The Sertoli cells line the seminiferous tubules in the testicles along with primitive germ cells. The principal role of the Sertoli cells is to provide germ cell support, initiate and sustain spermatogenesis, and regulate pituitary gland function. Leydig cells are responsible for the production of testosterone necessary for spermatogenesis.7,8
The hypothalamic-pituitary-gonadal (HPG) axis is a complex system that regulates gonadal and sexual function. The hypothalamus is the integrative center for the reproductive hormonal axis; it secretes gonadotropin-releasing hormone (GnRH), which releases luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary. After release from the pituitary, LH interacts with receptors on the Leydig cell membrane in the testes to synthesize and secrete testosterone. FSH binds to Sertoli cell membrane receptors to initiate and maintain spermatogenesis. Synthesis and release of gonadotropins are regulated by neuroendocrine signals from the central nervous system.7,8
Human reproduction requires fertilization of a mature ovum through introduction of sperm-containing semen via the vagina. During this event, penile erection and ejaculation are essential. The parasympathetic nervous system controls erectile function while the sympathetic nervous system is responsible for emission and ejaculation. The seminiferous tubules, a tubular network within the testes, carry seminal fluid containing mature spermatozoa to the epididymis through the vas deferens to the ejaculatory ducts and into the urethra. Periurethral muscle contractions expel the seminal fluid out of the urethra and into the female reproductive tract.7,8
Etiology
Azoospermia is defined as the absence of spermatozoa in the seminal fluid. Causes of infertility in the azoospermic male may be categorized as pretesticular, testicular, or post-testicular.
Pretesticular Deficiency: As a less common etiology than other causes of male infertility, hypogonadotropic hypogonadism (HH) is caused by insufficient GnRH and/or FSH and LH secretion.2 These insufficiencies result in deficient androgen secretion and spermatogenesis. HH can arise from congenital GnRH deficiency, hemochromatosis, genetic disorders, pituitary and hypothalamic tumors, hormonal abnormalities, or medications. In addition, systemic disorders such as chronic illnesses, nutritional deficiencies, and obesity have been identified as causes of HH.9
Testicular Deficiency: Testicular deficiency, sometimes referred to as nonobstructive azoospermia, is spermatogenic failure caused by conditions other than obstruction or HPG dysfunction. This category of dysfunction can be further subdivided into congenital, acquired, or idiopathic testicular failure. Congenital failure can manifest as anorchia, testicular dysgenesis, cryptorchidism, or genetic abnormalities. Acquired testicular failure can be the result of trauma, testicular torsion, orchitis, exogenous factors (e.g., medications, systemic diseases, varicocele), or surgeries that damage the vascular structure of the testes.3 About 15% of the general male population and approximately 40% of men presenting with male infertility have varicoceles.10
Post-testicular Deficiency: Often referred to as obstructive azoospermia, post-testicular deficiency is due to either ejaculatory dysfunction or obstruction of sperm delivery. This form of male infertility is less common than non-obstructive azoospermia, but occurs in approximately 40% of men presenting with azoospermia.2 The obstruction can arise from the epididymis, vas deferens, or ejaculatory duct and can be acquired or congenital.3
Idiopathic Infertility: In approximately 30% to 40% of men who are infertile, no male infertility factor can be identified.3 These men frequently have no previous history of infertility, unremarkable physical examination, and normal endocrine laboratory evaluation. Semen analysis reveals a decreased number of spermatozoa, reduced sperm motility, and many abnormal forms of sperm. These findings commonly occur together and are termed oligo-astheno-teratozoospermia or OAT syndrome. Idiopathic male infertility can be attributed to endocrine disruption due to environmental pollution, reactive oxygen species, or genetic abnormalities.3
Medications and Lifestyle Factors
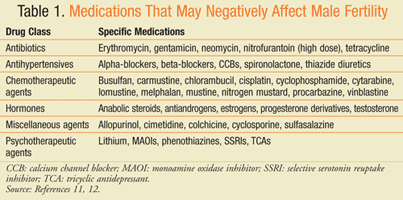
Numerous substances have been implicated as causes of infertility. Establishing cause and effect of medications is difficult due to confounding factors and small sample sizes. Drugs may affect male infertility through direct gonadotoxic effects, alteration of the HPG axis, impairment of ejaculation and/or erectile function, and changes in libido.11 Recreational and illicit drugs including alcohol, tobacco, marijuana, cocaine, and amphetamines are cited as causes of infertility. A number of medications have been identified as causes of male infertility including chemotherapy, antihypertensives, hormones, psychotropics, antidepressants, and antibiotics (TABLE 1).11,12 Testosterone replacement therapy is a common medical etiology of male factor infertility and has an adverse effect on spermatogenesis. In addition, many vaginal lubricants have been shown to inhibit sperm motility and velocity potentially leading to infertility. Hydroxyethylcellulose-based lubricants, mineral oil, and canola oil lack these effects on sperm and may be recommended as alternatives.13 A pharmacist can review the medication profile of patients presenting with infertility to identify potential drug therapy causes and reduce the need for further evaluation.
Evaluation
The American Urological Association (AUA) recommends an initial screening evaluation of the male partner of an infertile couple if pregnancy has not occurred within one year of regular, unprotected intercourse. An earlier evaluation is reasonable if a known infertility factor exists or a male doubts his fertility potential.2 A previous history of fertility does not exclude the possibility of secondary infertility. Men with secondary infertility are evaluated in the same manner as men who have never initiated pregnancy. The female partner should also undergo evaluation during this time period.
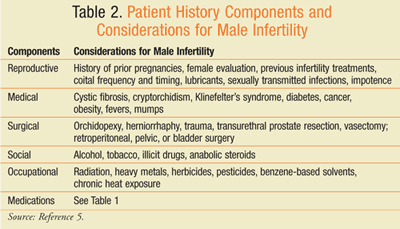
The basic components for the evaluation of male infertility include a detailed review of patient history (TABLE 2), physical examination, at least two semen analyses, and hormonal assessment of the HPG axis. The patient’s history may identify risk factors and/or behavior patterns that affect fertility potential. During the physical examination, particular attention is given to body habitus and secondary sex characteristics. The scrotum, testes, and prostate are evaluated for signs of infection, obstruction, or varicocele. Additional tests include transrectal or scrotal ultrasonography, post-ejaculatory urinalysis, and genetic testing, which are obtained on an individual basis when indicated.
Semen analysis is the fundamental laboratory test in the evaluation of male infertility.14 It provides information about semen quality and volume, sperm concentration, motility, and morphology. These results may be compared with reference ranges and used to identify men with abnormal semen parameters who may benefit from ART such as intrauterine insemination (IUI) or in vitro fertilization (IVF) with or without intracytoplasmic sperm injection (ICSI). Men with normal semen analyses rarely have sperm that contribute to infertility.1
Treatment
Some infertile men will have medically or surgically correctable causes, making natural conception possible with appropriate intervention. Treatment is dependent on the underlying etiology and thus requires an accurate diagnosis for proper treatment. If a treatable or correctable infertility factor is identified, it should be corrected using appropriate medical or surgical therapies. Men with uncorrectable, untreatable, or unknown etiologies may benefit from ART.
ART procedures have been used in the U.S. since 1981 and have dramatically improved fertility rates. IVF-ICSI is a type of ART used to overcome severe oligospermia or azoospermia by injecting a single sperm into the cytoplasm of a mature egg. IVF-ICSI is a treatment option for males with azoospermia, yielding live birth rates comparable to those achieved with IVF without ICSI.15 Azoospermic men require microdissection testicular sperm extraction to surgically retrieve sperm that may be usable for IVF-ICSI; the couple must be counseled on the success rates of sperm harvest as well as IVF-ICSI success rates using this sperm. The risks associated with IVF-ICSI include ovarian hyperstimulation syndrome, multiple gestation, perinatal complications, and genetic disorders.16 Some couples undergo ART without an appropriate evaluation by an infertility specialist; this is dangerous because approximately 6% of males evaluated have a serious underlying medical condition.5,17
Pretesticular Deficiency: Hyperprolactinemia is the most common endocrine disorder of the hypothalamic-pituitary axis and is a known cause of HH. Fertility may be restored by normalizing prolactin serum concentrations. Several drugs including dopamine antagonists, selective serotonin reuptake inhibitors, tricyclic antidepressants, and high-dose estrogen therapy cause hyperprolactinemia and should be discontinued if possible in this situation.18 A dopamine agonist such as cabergoline or bromocriptine is the treatment of choice for most patients with hyperprolactinemia.18
Gonadotropins may be used to treat HH caused by hypothalamic or pituitary disease not associated with hyperprolactinemia. Human chorionic gonadotropin (hCG) has biological activity similar to LH but has a longer half-life. It is usually initiated alone at 1,500 to 2,000 IU 3 times per week given intramuscularly or subcutaneously in the thigh for 18 to 24 weeks and then titrated at 2-week intervals to achieve serum testosterone concentrations between 300 and 500 ng/dL. Seminal fluid is evaluated for spermatogenesis every 2 to 4 weeks. If sperm concentrations are unsatisfactory after 6 to 12 months of therapy, an FSH preparation should be added to hCG. Men using hCG should be warned of possible side effects such as gynecomastia, headaches, and mastalgia.19
FSH is available as human menopausal gonadotropin (hMG) or recombinant human FSH (r-hFSH). hMG contains purified extracts of LH and FSH, while r-hFSH consists of only FSH. It is preferred because of similar efficacy and substantially lower costs when compared with r-hFSH. The initial dose of hMG is 75 IU administered 3 times weekly and may be titrated to 150 IU. Although the hMG is generally well tolerated, headache, mastalgia, and injection site reactions have been reported.20
Pulsatile GnRH therapy is an off-label use for the treatment of HH caused by hypothalamic disease. GnRH is administered via a subcutaneous pump that delivers GnRH over 60 minutes every 2 hours. The typical starting dose is 25 ng/kg/bolus titrated based on serum testosterone levels at 2-week intervals. Once target testosterone levels are achieved, the dose is held constant; testicular volume and seminal fluid analyses are performed each month. Catheter-site complications such as phlebitis, hematoma, and infection occur in approximately 7% of patients.21 Men receiving GnRH therapy should be instructed by their health care professional to monitor the needle-insertion site daily and report any redness, pain, or drainage.
Testicular Deficiency: Hypergonadotropic hypogonadism is due to dysfunctional testes and is characterized by elevated gonadotropins, low testosterone, and oligospermia or azoospermia. This is also known as primary testicular failure. These patients rarely achieve paternity through natural conception due to seminiferous tubule damage.22 Gonadotropin therapy is not effective under this circumstance and IVF-ICSI is typically necessary for successful fertilization.
The treatment of varicocele has traditionally been considered controversial, and no consensus about the efficacy of interventions exists. Results from a recent randomized, controlled trial suggest that varicocelectomy in men with palpable varicocele(s) improves semen parameters and increases the odds of spontaneous pregnancy within 1 year when compared with observation.23 The AUA suggests varicocele repair be offered to the male partner of a couple attempting to conceive when the varicocele is palpable, the couple has documented infertility, the female is fertile, and the male partner has one or more abnormal semen or sperm parameters. IVF with or without ICSI may be considered when there is the need to treat a female infertility factor, regardless of the presence of varicocele and suboptimal semen quality.10
Post-testicular Deficiency: Treatment options for infertility due to obstructive azoospermia include IVF-ICSI and microsurgical procedures (vasectomy reversals).24 Ejaculatory dysfunction, more specifically failure of emission, can be treated with alpha-adrenergic agonists that facilitate ejaculation.25 This approach can convert a patient with failed emission to retrograde ejaculation and sperm can then be retrieved for insemination.
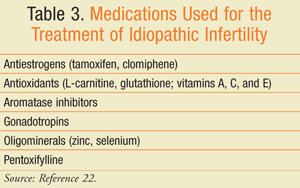
Idiopathic Infertility: There is no consensus on the correct management of idiopathic infertility. A variety of empiric medical therapies have been attempted to improve pregnancy rates (TABLE 3) despite a lack of proven efficacy. According to an AUA survey of fertility specialists, clomiphene citrate, hCG, and anastrazole are the most commonly prescribed medications for idiopathic male infertility.26
Summary
Infertility affects 8 million couples in the U.S., with many requiring medical intervention to achieve pregnancy. It is essential that both partners be thoroughly evaluated for causes to optimize treatment and minimize morbidity associated with possible underlying medical conditions. The pharmacist’s role in health care affords the opportunity to identify medications with potential adverse effects on fertility, recommend alternative drugs, and counsel patients on the proper use of medications. Advances in ART have improved outcomes for severely infertile couples, but further research is needed to better understand unknown causes of male infertility and to develop more effective treatments.
REFERENCES
1. American Urological Association. The optimal evaluation
of the infertile male: AUA best practice statement. Revised 2010.
www.auanet.org/content/media/optimalevaluation2010.pdf. Accessed
February 14, 2012.
2. American Urological Association. The evaluation of the
azoospermic male: AUA best practice statement. Revised 2010.
www.auanet.org/content/media/azoospermicmale2010.pdf. Accessed February
6, 2012.
3. European Association of Urology. Guidelines on male
infertility. Updated April 2010.
www.uroweb.org/gls/pdf/14_Male_Infertility%202010.pdf. Accessed November
29, 2011.
4. Frey KA. Male reproductive health and infertility. Prim Care Clin Office Pract. 2010;37:643-652.
5. Kim HH, Schlegel PN, Goldstein M. Infertility: the male. In: Legato MJ, ed. Principles of Gender-Specific Medicine. 2nd ed. Amsterdam, Netherlands: Elsevier; 2010:366-380.
6. Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics. 2011;66:691-700.
7. Molina PE. Chapter 8. Male reproductive system. In: Molina PE, ed. Endocrine Physiology. 3rd ed. New York, NY: McGraw-Hill; 2010.
8. Snyder PJ, Matsumoto AM. Male reproductive physiology. Updated February 3, 2010. UpToDate. www.uptodate.com. Accessed January 30, 2012.
9. Fode M, Sønksen J, McPhee SJ, Ohl DA. Chapter 23. Disorders of the male reproductive tract. In: McPhee SJ, Hammer GD, eds. Pathophysiology of Disease. 6th ed. New York, NY: McGraw-Hill; 2010.
10. American Urological Association. Report on varicocele
and infertility: an AUA best practice policy and ASRM practice committee
report. April 2011.
www.auanet.org/content/media/varicoceleinfertility.pdf. Accessed
February 6, 2012.
11. Nudell DM, Monoski MM, Lipshultz LI. Common medications and drugs: how they affect male infertility. Urol Clin N Am. 2002;29:965-973.
12. Brugh VM 3rd, Matschke M, Lipschultz LI. Male factor infertility. Endocrinol Metab Clin North Am. 2003;32:689-707.
13. Practice Committee of the American Society of
Reproductive Medicine in collaboration with the Society for Reproductive
Endocrinology and Infertility. Optimizing natural fertility. Fertil Steril. 2008;90(suppl 3):S1-S6.
14. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th ed. Cambridge, UK: Cambridge University Press; 1999.
15. Practice Committee of the American Society for
Reproductive Medicine; Practice Committee of the Society for Assisted
Reproductive Technology. Genetic considerations related to
intracytoplasmic sperm injection (ICSI). Fertil Steril. 2006;86(5 suppl 1):S103-S104.
16. Alukal JP, Lamb DJ. Intracytoplasmic sperm injection (ICSI)—what are the risks? Urol Clin North Am. 2008;35:277-288.
17. Alukal JP, Lipshultz LI. Why treat the male in the era of assisted reproduction? Semin Reprod Med. 2009;27:109-114.
18. Mah PM, Webster J. Hyperprolactinemia: etiology, diagnosis, and management. Semin Reprod Med. 2002;20:365-373.
19. Sokol RZ. Endocrinology of male infertility: evaluation and treatment. Semin Reprod Med. 2009;27:149-158.
20. Howles CM, Tanaka T, Matsuda T. Management of male hypogonadism. Semin Reprod Med. 2002;20:327-338.
21. Gonadorelin acetate. Clinical Pharmacology [database online]. www.clinicalpharmacology-ip.com.cuhsl.creighton.edu/Default.aspx. Accessed February 24, 2012.
22. Isidori A, Maurizio L, Romanelli F. Treatment of male infertility. Contraception. 2005;72:314-318.
23. Abdel-Meguib TA, Al-Sayaad A, Tayib A, Farsi HM. Does
varicocele repair improve male infertility? An evidence-based
perspective from a randomized, controlled trial. Eur Urol. 2011;59:455-461.
24. American Urological Association. The management of
obstructive azoospermia: AUA best practice statement. Revised, 2010.
www.auanet.org/content/media/obstructiveazoospermial2010.pdf. Accessed
February 6, 2012.
25. Howards SS. Treatment of male infertility. N Engl J Med. 1995;332:312-317.
26. Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES. Empirical
medical therapy for idiopathic male infertility: a survey of the
American Urological Association. J Urol. 2012;187:981-986.
To comment on this article, contact rdavidson@uspharmacist.com.





