ABSTRACT: The CDC’s Guideline for Prescribing Opioids for Chronic Pain is intended for clinicians prescribing opioids for chronic pain outside of active cancer treatment, palliative care, and end-of-life treatment. The guideline provides recommendations on when to initiate or continue opioids for chronic pain; opioid selection, dosage, duration, follow-up, and discontinuation; and assessing risk and addressing harms of opioid use. Chronic pain should primarily be managed by non-pharmacologic or nonopioid pharmacologic therapy. When utilized for pain management, opioids should be started at the lowest effective dosage and titrated slowly. Prescribers and patients are mutually responsible for mitigating risks associated with opioid use.
US Pharm. 2017;42(10):HS-31-HS-34.
Approximately 11% of the adult population in the United States is affected by chronic pain, which can have a significant impact on an individual’s daily function. Increasingly, opioids have been prescribed to diminish the effects of pain.1 Opioid overdoses and related deaths have reached record levels in the U.S., and the problem is often described as an epidemic.2
When compared with other treatment options for chronic pain, it is becoming evident that opioids carry considerable risks and have uncertain benefits.3 Current evidence does not support the safety, efficacy, and economic benefits of long-term opioid therapy—more research is needed to fill these crucial gaps.3
Primary-care providers express concern about the misuse of opioid pain medications and patient addiction, have difficulties handling patients with chronic pain, and report a lack of training in prescribing opioids.4 Despite the upward trend in the number of opioid prescriptions written, no guidelines discussing optimal strategies for treating chronic pain with these potentially harmful medications were available until recently.1
Development of Pain Management Guidelines
To improve patient safety for those with chronic pain and to address the current prescription opioid overdose epidemic, the CDC officially published the Guideline for Prescribing Opioids for Chronic Pain in March of 2016. The CDC is the first agency at the federal level to provide practical recommendations to primary care clinicians regarding the role of prescription opioids for chronic pain in adult patients outside of active cancer, end-of-life, or palliative care.2,4,5
In creating the guideline, the agency undertook a rigorous review of the best available scientific evidence and included input from experts including nationally recognized researchers, providers, and partners. Evidence consisted of observational studies as well as randomized clinical trials, with notable limitations. The revision process also included scientific review, expert review and input, peer review, public comment, and federal advisory committee review. The final report provides primary care clinicians (e.g., family physicians, internists, nurse practitioners, and physician assistants) the essential tools needed to address safer opioid prescribing and monitoring.2,4,5 Pharmacists and pain specialists should be involved as allowed by state regulations in implementing these guidelines.4
The guideline provides 12 major recommendations for safe and effective use of opioids in the treatment of patients with chronic pain (i.e., pain conditions that typically last more than 3 months or past the time of normal tissue healing) in outpatient settings. The recommendations in this guideline are not meant to be prescriptive standards, but are considered voluntary.4 These 12 recommendations are summarized in Tables 1 to 3.
Determining When to Initiate or Continue Opioids
for Chronic Pain
Opioids should not be considered first-line or routine therapy for chronic pain (Table 1). Although long-term benefits of both opioid and nonopioid therapies are limited, nonopioid management is preferred because of the lower associated risks. Nonpharmacologic treatment such as exercise therapy and cognitive behavioral therapy should be recommended for patients with chronic pain to decrease their pain and improve functional ability. These measures should be combined with nonopioid pharmacologic therapy such as nonsteroidal anti-inflammatory drugs, acetaminophen, anticonvulsants, and serotonin-norepinephrine reuptake inhibitors when benefits outweigh risks. Patients should be encouraged to be active participants in their care plans.4
Before starting opioid therapy, benefits and risks should be assessed. Prior to initiation of therapy, healthcare providers should establish treatment goals and develop plans to evaluate the effectiveness of treatment. If patients are established on an opioid regimen, goals should incorporate the expected results of continued treatment. Improvement not only in pain relief, but also in function, should be measured and included in goals. Clinicians should confirm that treatment for depression, anxiety, or other psychological comorbidities is optimized; these conditions often coexist with and can prevent the resolution of pain.4
Because of the significant risks associated with opioids, the decision to initiate these medications should be carried out only with full understanding of these risks by both the prescriber and the patient. Discussions regarding the risks and realistic benefits of opioids should occur regularly and should stress the responsibilities of the patient as well as the healthcare provider in mitigating risk.1,3
Opioid Selection, Dosage, Duration,
Follow-up, and Discontinuation
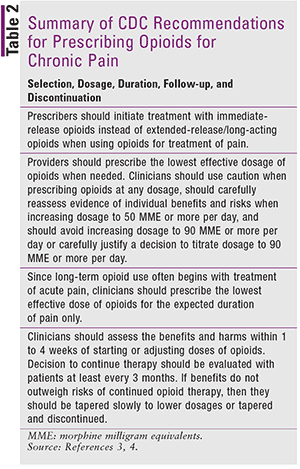
When selecting an opioid, immediate-release formulations are safer than extended-release or long-acting opioids, regardless of whether the drug is used for acute or long-term treatment (Table 2). The FDA has relabeled long-acting opioids for use in severe, long-term cases when immediate-release opioids and other options are ineffective or are not tolerated after a trial of at least 1 week.1
When starting opioids for chronic pain, the lowest effective dosage should be prescribed and titrated slowly; “start low and go slow” is the rule of thumb.3,4 Dosages greater than 50 morphine milligram equivalents (MME) should be prescribed with caution and carefully reassessed at every visit. Evidence supporting the prescribing of high doses (90 MME or more) is insufficient; such doses should be avoided because of an increased risk of overdose at higher dosages and known challenges associated with opioid tapering.1,4 The tools and resources provided by the CDC on their website (www.cdc.gov/drugoverdose/prescribing/resources.html) can aid in determining the total daily amount of opioid use.4,6,7
Healthcare providers should also carefully consider the use of opioids for acute pain. Since long-term opioid use often begins with acute pain treatment, the shortest duration of immediate-release opioids should be prescribed. Treatment for 3 or fewer days is often sufficient for most patients and more than 7 days is rarely required.4
When opioids are initiated or when doses are escalated, the benefits and risks should be reassessed within 1 to 4 weeks; if therapy is continued, it should be reevaluated with patients at least every 3 months. If expected benefits do not outweigh potential harms, then clinicians may consider tapering of opioids to lower dosages or discontinuing therapy and pursuing preferred nonopioid interventions.1,4
Assessing Risk and Addressing Harms
of Opioid Use
Risk factors for opioid-related harms, such as overdose, should be evaluated before initiating, and periodically during, opioid therapy (Table 3). Overdose risk increases in a dose-response manner; risk doubles at 50 to 99 MME/day and increases by a factor of up to nine at greater than 100 MME/day.3 Management strategies to mitigate risk, such as offering naloxone to patients at high risk for opioid overdose, should be implemented.4
Prescription-drug monitoring programs (PDMPs) operated by the states contain information on the amount and types of controlled substances a patient has received. The data from PDMPs can be utilized to determine the amount of opioid being consumed by a patient. Clinicians should review PDMP data, if available, at the start of therapy as well as throughout therapy to help determine if the patient is actually using the opioid as prescribed or if there are any dangerous combinations that put them at high risk for overdose. Urine drug testing before starting opioid therapy and at least yearly to assess for prescribed opioids, other controlled prescription drugs, and illicit drugs can also be a useful tool for physicians in managing opioid use. Benzodiazepine use with opioids should be avoided whenever possible.1,4
For patients with opioid-use disorder, prescribers should offer evidence-based treatment options such as buprenorphine, naltrexone, or methadone in combination with behavioral therapies. If an opioid-use disorder is suspected, concerns should be discussed with the patient. It is recommended not to ask patients with substance-use disorder to leave their healthcare provider’s practice, as they may perceive it as abandonment, which could also jeopardize their safety.1,4
Role of the Pharmacist
In an inpatient setting, pharmacists can help ensure safe and appropriate use of opioids through pain medication reconciliation at admission/discharge and monitoring/managing adverse events that are due to opioid use. In both inpatient and outpatient settings, patient assessments can be conducted by pharmacists, and subsequent recommendations for dose adjustments can be provided to the clinicians involved in managing pain regimens. Pharmacists can also play a key role in improving outcomes by providing education to the patient and their family members.8,9
Conclusion
Treating chronic pain is challenging. The use of opioids as first-line therapy for chronic pain is not recommended. The known severe and potentially fatal risks outweigh the unverified and temporary benefits of opioids for chronic pain.
There are very few resources available for primary care practitioners regarding chronic pain management outside of cancer, palliative, or end-of-life care. The CDC guideline summarized in this article was developed as such a resource. In addition, there are several user-friendly resources and tools available on the CDC website for clinicians, as well as patients, to help with implementation of the guidelines. Although more research is needed to support the recommendations, this is a step in the right direction and has the potential to save many lives.
REFERENCES
1. Bredemeyer M. CDC develops guideline for opioid prescribing. Am Fam Physician. 2016;93(12):1042-1043.
2. Olsen Y. The CDC guideline on opioid prescribing: rising to the challenge. JAMA. 2016;315(15):1577-1579.
3. Frieden TR, Houry D. Reducing the risks of relief—the CDC opioid-prescribing guideline. N Engl J Med. 2016;374(16):1501-1504.
4. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. Erratum in: MMWR Recomm Rep. 2016;65(11):295. www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm.
5. Houry D, Baldwin G. Announcing the CDC guideline for prescribing opioids for chronic pain. J Safety Res. 2016:83-84.
6. Alliant Quality. Medication safety resources. www.alliantquality.org/sites/default/files/ApplyingtheNewCDCGuidelineforPrescribingOpioids 2011SOW-GMCFQIN-C361-16-05(1).pdf. Accessed August 30, 2017.
7. CDC. Injury prevention and control: opioid overdose. www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed August 30, 2017.
8. Ghafoor VL, Phelps P, Pastor J. Implementation of a pain medication stewardship program. Am J Health Syst Pharm. 2013;70(23):2070,2074-2075.
9. Norman JL, Kroehl ME, Lam HM, et al. Implementation of a pharmacist-managed clinic for patients with chronic nonmalignant pain. Am J Health Syst Pharm. 2017;74(16):1229-1235.
To comment on this article, contact rdavidson@uspharmacist.com.