US Pharm. 2013;8(38):22-26.
ABSTRACT: A prevalent complication of chronic kidney disease (CKD), especially in dialysis patients, iron deficiency anemia (IDA) continues to be underdiagnosed and undertreated. The main causes of IDA in this population are reduced intake and impaired intestinal absorption of dietary iron, blood losses, chronic inflammation, and increased iron requirements during therapy with erythropoiesis-stimulating agents (ESAs). The addition of IV iron can relieve iron-restricted erythropoiesis and improve ESA response, as well as balance the risks of ESA therapy. Clinical guidelines for anemia of CKD include recommendations for the use of IV iron. There are a number of factors to consider when managing anemia of CKD, and the various forms of iron are not identical.
A prevalent complication of chronic kidney disease (CKD), particularly in dialysis patients, iron deficiency anemia (IDA) remains underdiagnosed and undertreated. This article presents an update on the diagnosis of IDA and the management of IDA with various forms of IV iron.
Introduction
The World Health Organization defines anemia as a hemoglobin (Hgb) level of <13 g/dL in men and <12 g/dL in women.1 In the United States, iron deficiency is the most common cause of anemia. A deficiency of iron in RBCs reduces tissue oxygen delivery, increases cardiac output, and may result in ventricular dilation and hypertrophy, if left untreated.2 The main causes of iron deficiency are increased demand for iron, iron loss, and—frequently the case in CKD—decreased iron stores, reduced iron absorption, or inflammatory iron block.
Anemia of CKD may be due to insufficient production of erythropoietin by diseased kidneys, diminished RBC survival, iron or vitamin deficiencies, bleeding diathesis, hyperparathyroidism, chronic inflammation, or comorbidities.2,3 Iron deficiency also can develop in hemodialysis (HD) patients receiving erythropoiesis-stimulating agents (ESAs), which cause an increased demand for iron; blood loss from dialysis is another cause of iron deficiency.4 Diabetic patients are at increased risk for anemia, and anemia itself is an independent risk factor for CKD.5,6
Symptoms of iron deficiency include fatigue, weakness, anorexia, insomnia, angina, tachycardia, dyspnea, decreased mental and physical performance, and possibly heart failure. Although anemia correction in CKD has been shown to slow the progression of renal disease and improve overall quality of life, IDA remains underdiagnosed and undertreated.2,7,8
Because both early-stage CKD and anemia are usually asymptomatic, renal and hematologic laboratory values should be monitored annually in patients at risk.2,3,8 As glomerular filtration rate (GFR) decreases with progressive CKD, the risk of anemia increases from approximately 27% in stage 1 CKD to 76% in stage 5 (GFR <15 mL/min).5,9
Laboratory Tests
Transferrin saturation (TSAT) and ferritin values help determine the presence of IDA (TABLE 1). Absolute IDA—in which TSAT is <20% and ferritin is <100 ng/mL—refers to the depletion of iron stores that occurs when there is insufficient iron to produce Hgb. Functional IDA (elevated ferritin and TSAT <20%) may develop during ESA therapy owing to inadequate mobilization despite sufficient stores to keep up with RBC production during erythropoiesis.10,11 Failure to reach target Hgb levels may occur despite large ESA dosages.
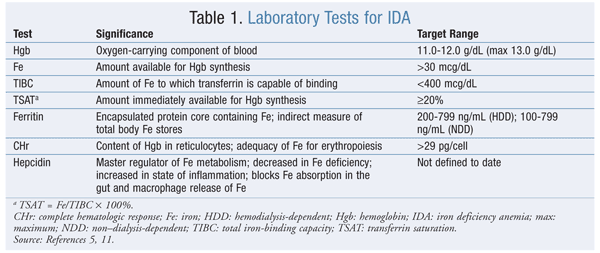
Ferritin, a nonspecific acute-phase reactant, is elevated in chronic inflammation. In the presence of CKD, ferritin cutoff values for both predialysis and HD are higher in functional IDA, at >100 ng/mL and >200 ng/mL, respectively. See TABLE 1 for other monitoring parameters (e.g., complete hematologic response) used during iron therapy.5,11
Iron and ESA Therapy
Despite its benefits, ESA therapy is associated with an increased risk of adverse drug events (ADEs) such as cardiovascular (CV) complications, hypertension, RBC aplasia, and development of antierythropoietin antibodies. Three trials (CHOIR, CREATE, and TREAT) raised concerns about ESA therapy and its associated CV and mortality risks because of the higher Hgb levels in, and/or higher ESA doses administered to, hyporesponders.12-14 IV iron as an adjunct to ESA therapy has become standard treatment for optimizing Hgb status and reducing ESA dosing requirements. Up to a 70% reduction in required ESA dosage has been noted in patients receiving IV iron.15,16 However, iron underutilization has been reported in anemic CKD patients receiving ESA therapy.17
Iron Supplementation
ADEs, drug interactions, and lack of adherence limit the use of oral iron therapy. For HD-dependent patients, who often have limited absorption of oral iron, IV supplementation is preferred.18 The 2006 and 2007 updates to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines for anemia of CKD recommend that HD patients on ESA therapy receive IV iron supplementation to maintain a target Hgb of 11 g/dL to 12 g/dL (not to exceed 13 g/dL). The guidelines also recommend that non–dialysis-dependent and peritoneal dialysis–dependent patients receive iron either orally or IV.19,20
Iron repletion should begin before initiation of ESA therapy. Iron levels should initially be monitored monthly until iron stores are replenished, then monitored quarterly. Smaller maintenance doses may be necessary after repletion, because Hgb optimization can occur in patients whose ferritin is >500 ng/mL. Overall clinical status, degree of ESA responsiveness, and other causes of increased ferritin (infection, inflammation) should be considered. Data are lacking regarding cessation of IV iron therapy. An increase in Hgb >1 g/dL over 1 month indicates a therapeutic response to iron therapy when IDA diagnosis in complex situations is unclear. Monitoring of iron levels also is important to avoid iron overload or toxicity and to prevent hemochromatosis from developing.5
Pharmacokinetics of IV Iron Formulations
IV iron formulations contain an iron core stabilized by a carbohydrate outer shell that maintains iron in a colloid form and controls its release. Formulations differ in iron core size and in the type and density of the surrounding carbohydrate shell. Pharmacokinetics differs across iron agents, with an inverse relationship between the strength of the iron complex and the rate of iron release. The stronger the complex, the more slowly bioactive iron is released, which may lead to free iron toxicity because of its lower potential to saturate transferrin.20 Iron-carbohydrate complexes in plasma are phagocytosed by the reticuloendothelial system, which degrades the carbohydrate shell; iron is stored as ferritin or transported out of the cell by an export protein. The exported iron binds to free sites on plasma transferrin, which delivers iron to transferrin receptors, and is then utilized in the formation of Hgb. Unutilized iron gets stored and, when needed, is released from the cell to undergo transferrin recycling.
Comparison of IV Iron Products
IV iron products (see TABLE 2 for a comparison) are either dextran-containing or non–dextran-containing.21 ADE profiles of the various IV iron formulations are based on differences in molecular size, degradation kinetics, and bioavailability.22 There are concerns surrounding iron’s safety.
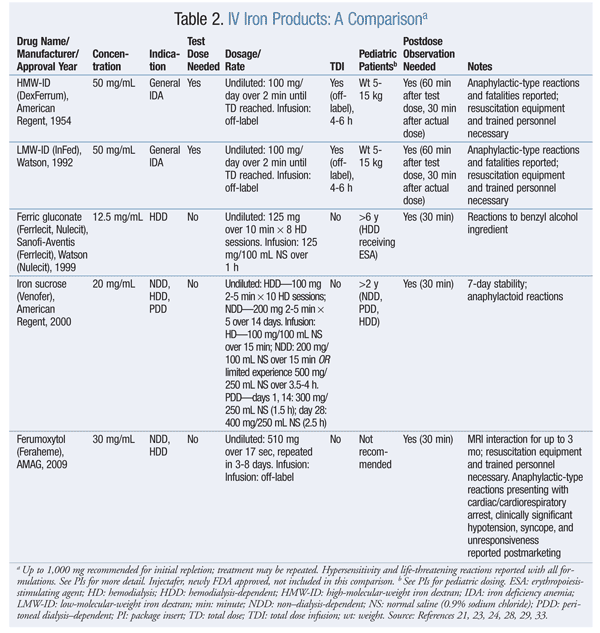
Iron dextran (ID) formulations carry a black box warning about fatal anaphylactic reactions, likely because of antibodies to the iron-carbohydrate or ID complex or the dextran component, particularly with high-molecular-weight ID (HMW-ID). The introduction of low-molecular-weight ID (LMW-ID) substantially reduces the risk of anaphylaxis. With ID therapy, test doses are required, along with an observation period for antibody reactions.23,24
Newer IV irons—ferric gluconate and iron sucrose—do not contain dextran and have a better safety profile. Chertow et al compared absolute rates of life-threatening ADEs reported by the FDA from 2001 to 2003. 25 For four different parenteral iron preparations (iron sucrose, ferric gluconate, LMW-ID, and HMW-ID), ADEs were 0.6, 0.9, 3.3, and 11.3 per million patients, respectively.
Vasoactive reactions, which are due to the appearance of nontransferrin-bound or free (labile) iron in the circulation, include a drop in blood pressure, acute edema of extremities, and acute onset of diarrhea when large IV iron doses are administered rapidly.26 Small undiluted or infusion doses of LMW-ID are recommended; a total dose infusion (TDI) of 1,000 mg is possible, but it must be infused over 4 to 6 hours to avoid vasoactive reactions.27 Also, IV steroid premedication has successfully prevented these reactions.
TDI of iron sucrose and ferric gluconate is not recommended because the potential for vasoactive reactions and gastrointestinal symptoms increases with large doses. Smaller undiluted or infusion doses are recommended. Iron sucrose has broader FDA-labeled indications than does ferric gluconate (TABLE 2).28-31
Ferumoxytol is the newest IV iron formulation to enter the U.S. market. The total dose is administered undiluted in two separate injections. Although it contains dextran derivatives, no test dose is required. Comparative safety data with other IV iron formulations are lacking. Life-threatening anaphylactic-type reactions, cardiac/cardiorespiratory arrest, syncope, tachycardia/rhythm abnormalities, angioedema, ischemic myocardial events, congestive heart failure, absent pulse, and cyanosis have been reported for ferumoxytol.32,33 On July 25, 2013, the FDA approved ferric carboxymaltose (Injectafer), a new single-dose preparation.34,35 Soluble ferric pyrophosphate delivered via hemodiasylate is a new iron formulation in phase III development.36
There is concern about the increased risk of infection with IV iron use. Iron may serve as a growth factor for bacteria, inhibit phagocytosis, and increase oxidative stress. Withholding IV iron therapy in cases of acute infection may be prudent.37 If iron release exceeds binding capacity or if transferrin is oversaturated, toxic unbound iron acts as a catalyst in the formation of free radicals—believed to lead to coronary artery inflammation—and the development of atherosclerosis and long-term CV risk. Iron may also cause LDL oxidation, which is more damaging to the coronary arteries. Myocardial infarction occurring with the use of IV iron, hypothesized to be caused by elevated serum ferritin levels, also has been reported.38,39
Conclusion
Iron is a treatment option for anemia of CKD that can successfully balance the risks of ESA therapy. The KDOQI guidelines were updated in 2007 to reflect this. Pharmacists should be aware that there are a variety of factors to consider when managing anemia of CKD, and the various types of iron are not identical.
REFERENCES
1. World Health Organization. Nutritional anaemias. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1968;405:5-37.
2. Adamson JW, Longo DL. Anemia and polycythemia. In: Longo DL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
3. Lerma EV. Anemia of chronic disease and renal failure. Medscape.
http://emedicine.medscape.com/article/1389854. Accessed May 8, 2013.
4. Streja E, Miller JE, Nissenson AR. Intravenous iron versus
erythropoiesis-stimulating agents: friends or foes in treating chronic
kidney disease anemia? ACKD. 2009;16:143-151.
5. KDOQI; National Kidney Foundation. KDOQI clinical practice
guidelines and clinical practice recommendations for anemia in chronic
kidney disease. Am J Kidney Dis. 2006;47(5 suppl 3):S11-S145.
6. Astor BC, Muntner P, Levin A, et al. Association of kidney
function with anemia: the Third National Health and Nutrition
Examination Survey (1988-1994). Arch Intern Med. 2002;162:1401-1408.
7. Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating
anemia early in renal failure patients slows the decline of renal
function: a randomized controlled trial. Kidney Int. 2004;66:753-760.
8. Silverberg DS, Wexler D, Iaina A. The role of anemia in the
progression of congestive heart failure. Is there a place for
erythropoietin and intravenous iron? J Nephrol. 2004;17:749-761.
9. McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501-1510.
10. Irwin C, Moriarity-Suggs C. Anemia management in chronic and end-stage kidney disease. US Pharm. 2010;35(3)(Oncology/Hematology suppl):8-13.
11. Pasricha SR. Is it time for hepcidin to join the diagnostic toolkit for iron deficiency? Expert Rev Hematol. 2012;5:153-155.
12. Singh AK, Szczech L, Tang KL, et al; CHOIR investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-2098.
13. Drüeke TB, Locatelli F, Clyne N, et al; CREATE investigators.
Normalization of hemoglobin level in patients with chronic kidney
disease and anemia. N Engl J Med. 2006;355:2071-2084.
14. Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT investigators. A
trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease.
N Engl J Med. 2009;361:2019-2032.
15. Coyne DW. It’s time to compare anemia management strategies in hemodialysis. Clin J Am Soc Nephrol. 2010;5:740-742.
16. Coyne DW. A comprehensive vision for intravenous iron therapy. Am J Kidney Dis. 2008;52(6 suppl):S14-S20.
17. Hörl WH. Clinical aspects of iron use in the anemia of kidney disease. J Am Soc Nephrol. 2007;18:382-393.
18. Besarab A, Coyne DW. Iron supplementation to treat anemia in patients with chronic kidney disease. Nat Rev Nephrol. 2010;6:699-710.
19. KDOQI. KDOQI Clinical Practice Guideline and Clinical Practice
Recommendations for anemia in chronic kidney disease: 2007 update of
hemoglobin target. Am J Kidney Dis. 2007;50:471-530.
20. Auerbach M, Coyne D, Ballard H. Intravenous iron: from anathema to standard of care. Am J Hematol. 2008;83:
580-588.
21. Silverstein SB, Gilreath JA, Rodgers GM. Intravenous iron therapy: a summary of treatment options and review of guidelines. J Pharm Pract. 2008;21:431-443.
22. Yessayan L, Sandhu A, Besarab A, et al. Intravenous iron dextran
as a component of anemia management in chronic kidney disease: a report
of safety and efficacy. Int J Nephrol. 2013;2013:703038. doi:10.1155/2013/703038.
23. DexFerrum (iron dextran injection) product information. Shirley, NY: American Regent, Inc; 2008.
24. INFeD (iron dextran injection) product information. Morristown, NJ: Watson Pharma, Inc; September 2009.
25. Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. Update on adverse events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378-382.
26. Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12-33.
27. Bailie GR. Comparison of rates of reported adverse events associated with i.v. iron products in the United States. Am J Health Syst Pharm. 2012;69:310-320.
28. Ferrlecit (sodium ferric gluconate complex in sucrose injection)
product information. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; August
2011.
29. Venofer (iron sucrose injection) product information. Shirley, NY: American Regent, Inc; September 2012.
30. Coppol E, Shelly J, Cheng S, et al. A comparative look at the
safety profiles of intravenous iron products used in the hemodialysis
population. Ann Pharmacother. 2011;45:241-247.
31. Van Wyck DB, Bailie G, Aronoff G. Just the FAQs: frequently asked
questions about iron and anemia in patients with chronic kidney
disease. Am J Kidney Dis. 2002;39:426-432.
32. Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology American Soc Hematol Educ Program. 2010;2010:338-347.
33. Feraheme (ferumoxytol) product information. Lexington, MA: AMAG Pharmaceuticals, Inc; November 2011.
34. Macdougall IC. New anemia therapies: translating novel strategies from bench to bedside. Am J Kidney Dis. 2012;59:444-451.
35. Gozzard D. When is high-dose intravenous iron repletion needed? Assessing new treatment options. Drug Des Devel Ther. 2011;5:51-60.
36. SFP clinical development progress.
www.rockwellmed.com/bio-pharma-soluble-ferric-pyrophosphate-clinical-development.htm.
Accessed July 18, 2013.
37. Garneata L. Intravenous iron, inflammation, and oxidative stress: is iron a friend or an enemy of uremic patients? J Ren Nutr. 2008;18:40-45.
38. Hayat A. Safety issues with intravenous iron products in the management of anemia in chronic kidney disease. Clin Med Res. 2008;6:93-102.
39. Pai AB, Conner TA. Oxidative stress and inflammation in chronic kidney disease: role of intravenous iron and vitamin D. J Pharm Pract. 2008;21:214-224.
To comment on this article, contact rdavidson@uspharmacist.com.






