US Pharm. 2016;41(3):HS6-HS8.
ABSTRACT: Mucositis—the breakdown of epithelial cells in the gastrointestinal tract—is a common and painful side effect of treatment for many cancer patients. It can occur anywhere in the GI tract, but the oral cavity is a common site. Not only can mucositis be debilitating for patients, but it can also lead to delays or dose reductions in life-saving treatments. In general, the more intense the chemotherapy, the greater the risk of mucositis. Preventive strategies include good oral hygiene and rinses, whereas treatment strategies focus on the amelioration of pain. A stepwise treatment approach that includes bland oral rinses, topical anesthetics, and systemic analgesic agents is suggested based on clinical guidelines.
Mucositis is a common complication in which chemotherapy agents and/or radiation used to treat cancer causes a breakdown in the rapidly dividing epithelial cells of the gastrointestinal (GI) tract.1-3 Although mucositis can occur anywhere in the GI tract, a common site is the oral cavity.4
Background
Patients at high risk for mucositis include those undergoing myeloablative therapies for stem-cell transplantation, radiation for head and neck cancer, or multicycle chemotherapy regimens.1-4 In general, the more intense the chemotherapy treatment, the greater the risk of mucositis; cisplatin, doxorubicin, 5-fluorouracil (5-FU; bolus more than infusional), methotrexate, melphalan, and cyclophosphamide carry high risks.4 Patients with poor dental hygiene, poorly fitting dental prostheses (trauma), or low WBC counts also may be at higher risk for mucositis.1-4
Mucositis can be painful and debilitating; severe cases render patients unable to eat or to take oral medications.4-7 Severe cases may lead to delays or dose reductions in potentially life-saving chemotherapy or other treatments.4-7 Patients with mucositis typically experience oral pain; erythema; and difficulty eating, drinking, speaking, and opening the mouth.4-7 Mucositis can also cause complications such as systemic infections and result in increased healthcare costs for patients and institutions.4-7
The diagnosis of mucositis is generally based on clinical findings. After a diagnosis has been made, two main grading scales are used by clinicians to assess severity: the World Health Organization’s (WHO) Oral Toxicity Scale and the National Cancer Institute’s (NCI) Common Toxicity Criteria (TABLE 1).6,7
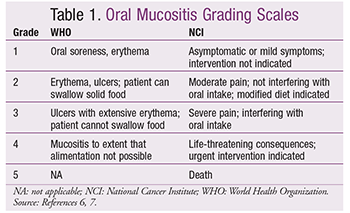
While mucositis is common, it is widely asserted that its true incidence in cancer patients is underreported. Mucositis of any degree is estimated to occur in approximately 40% of patients undergoing chemotherapy.1,2 The number increases to 75% in patients undergoing myeloablative therapies for hematopoietic stem cell transplantation (HSCT).3 In studies, the incidence of severe mucositis varies from 10% to 50%.1,2
Prevention
With mucositis, as with all complications of cancer treatment, the goal is to prevent it from occurring. Most preventive approaches focus on oral mucositis and generally include practicing good oral hygiene and keeping the mucosal area clean, dry, and free from possible sources of infection. With these goals in mind, clinicians may prescribe oral rinses, such as bicarbonate or saline-containing products, or magic mouthwash. Clinical trial data do not show superiority of one product over another, so guidelines suggest basing decisions on patient preference and clinical assessment.4 It is important to note that topical oral antimicrobial agents are not recommended by guidelines, except in cases when good oral hygiene is not possible.4,5 These therapies largely lack conclusive supportive data; however, many are still used in clinical practice.4 In particular, chlorhexidine is often used by prescribers but is not recommended in guidelines.4,5
One preventive approach with supportive trial data is cryotherapy with ice chips.8,9 The goal of such therapy is vasoconstriction of the vessels in the mouth, which decreases the delivery of cytotoxic chemotherapy to sensitive areas of the oral mucosa. Lilleby and colleagues randomized 40 patients with multiple myeloma who were undergoing high-dose melphalan therapy for HSCT to receive either cryotherapy or oral saline rinses 30 minutes before and 6 hours after therapy.8 Researchers then used the NCI scale to determine the development and severity of oral mucositis. Grade 3 mucositis occurred in three patients (14%) in the cryotherapy group and 14 patients (74%) in the group randomized to saline rinses (P = .0005); no grade 4 mucositis was reported.8
In a different trial (Svanberg and colleagues), 80 adults scheduled for HSCT were randomized to cryotherapy or standard oral care.9 Subjects receiving cryotherapy had significantly fewer days of IV opioids than those receiving oral care (0.77 ± 2.3 vs. 2.44 ± 4.6; P = .045). The cryotherapy group also had a lower mucositis score than the oral-care group on day 10 for autologous transplants (1.6 ± 1.9 vs. 4.3 ± 5.7; P = .042) and day 16 for allogeneic transplants (3.7 ± 1.8 vs. 11.6 ± 6.8; P = .021).9
Based on available data, the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) clinical practice guidelines recommend that cryotherapy with ice chips be used to prevent oral mucositis in patients receiving bolus 5-FU chemotherapy, as well as in patients receiving high-dose melphalan for HSCT conditioning.1
Palifermin—a keratinocyte growth factor that increases mucosal epithelium thickness—is FDA-approved to decrease the incidence and duration of severe oral mucositis associated with hematologic malignancies in patients who are receiving myelotoxic therapy requiring hematopoietic stem cell support.10 The MASCC/ISOO guidelines also suggest the use of palifermin in this specific patient population.1 Dosing of palifermin is 60 mcg/kg/day for 3 days before and 3 days after myelotoxic therapy (total of six doses).10
Palifermin has also been used in patients with head and neck cancer who are receiving concurrent chemotherapy and radiation. One study compared palifermin versus placebo in patients receiving cisplatin and radiotherapy for head and neck cancer.11 Patients in the palifermin arm reported a reduced oral mucositis pain-severity score and a decreased duration of severe mucositis (5 vs. 26 days). There were no between-group differences in the use of opioids for pain or in treatment-related adverse events, and progression-free survival was similar between groups.11
Treatment
If mucositis does occur, the goal remains to keep the area clean and free from possible infectious sources. Therefore, bland oral rinses and antiviral or antifungal medications may still be used.4 In addition, practical steps—such as changing all possible oral medications to IV forms—should be taken to minimize patient discomfort.
The main focus of mucositis treatment, however, should be adequate pain control and palliation of symptoms. With this in mind, the National Comprehensive Cancer Network (NCCN) Task Force on Prevention and Management of Mucositis in Cancer Care suggests a stepwise approach for the treatment of mucositis pain (FIGURE 1).4
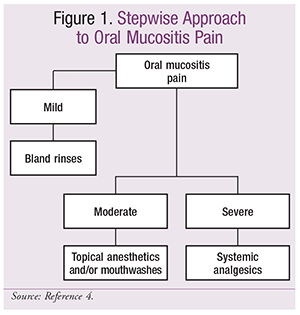
As mentioned above, the first step in treating mucositis pain is to use bland rinses or oral sodium bicarbonate rinses. In general, formulations containing alcohol should be avoided. If bland rinses fail and a patient progresses to moderate pain, the NCCN suggests the use of topical anesthetics, such as viscous lidocaine.4,12 These products are used topically to treat mucositis pain in the oral cavity, but many clinicians recommend that they not be swallowed, as the product may be systemically absorbed or may reduce the gag reflex and place the patient at risk for aspiration pneumonia. For the 2% viscous oral lidocaine product, manufacturer labeling recommends a dosage of 15 mL gargled no more frequently than every 3 hours, with a maximum of 4.5 mg/kg (or 300 mg per dose) and no more than eight doses per 24-hour period.12 Product labeling indicates that viscous lidocaine may be swallowed.12
In addition to topical lidocaine products, many institutions offer compounded magic mouthwash.4 These mouthwashes vary in formulation, but common ingredients include viscous lidocaine, diphenhydramine, sodium bicarbonate, nystatin, and sometimes steroids. No single formulation has been proven superior to the others.
The MASCC/ISOO guidelines suggest the use of morphine mouthwash as a topical treatment.1 Several studies have evaluated the effectiveness of morphine mouthwash in palliating oral mucositis pain. Cerchietti and colleagues published two studies examining morphine mouthwash for mucositis pain in patients receiving chemotherapy or radiotherapy.13,14 Both studies showed positive results. The second, and larger, of these studies included 26 patients with head and neck cancer who were randomized to receive either magic mouthwash (including diphenhydramine, lidocaine, and magnesium aluminum hydroxide) or 0.2% oral morphine rinses.14 In the morphine-rinse group, the duration of severe pain was 3.5 days less than that in the group receiving magic mouthwash (P = .032), and the intensity of oral pain was significantly lower (P = .038). Patients in this study also received pain medications in a stepwise fashion based on the WHO pain ladder (the first step being acetaminophen, the second being tramadol, and the third being opiates). No morphine-rinse patients required opiates for alleviation of mouth pain.14
Additional small studies have confirmed these results, including one by Sarvizadeh and colleagues that assessed 30 patients with head and neck cancer.15,16 Patients were randomized to magic mouthwash or morphine rinses.16 All 28 patients completing the study experienced a decrease in mucositis severity, with the morphine-rinse group showing a larger decrease at day 6 (P = .045). Morphine-rinse patients also reported being more satisfied with treatment.16
Another type of mouthwash studied for the treatment of mucositis pain and suggested in the MASCC/ISOO guidelines is doxepin.1 In small studies, this tricyclic antidepressant has been shown to possess anesthetic and analgesic properties when administered topically. A larger study by Leenstra and colleagues randomized 155 head and neck cancer patients receiving radiotherapy to doxepin mouthwashes or placebo.17 After the first dose of the allocated agent, patients crossed over to the opposite therapy on day 2.17 The crossover period was followed by continued use of the active agent.17 The primary endpoint was total pain reduction as an average of mouth and throat pain, which was measured by the numerical analogue scale of mouth pain in a questionnaire administered at baseline and at 5, 15, 30, 60, 120, and 240 minutes after the doxepin or placebo rinse.17 Reduction of mouth and throat pain was greater for doxepin than for placebo (P <.001).17
If topical therapies fail, systemic analgesia should be added. The MASCC/ISOO guidelines specify the use of fentanyl and morphine for mucositis pain.1 The NCCN adds that, in order to facilitate maximum pain control, medications should be scheduled rather than administered as needed.4 All systemic therapies should be chosen based on patient-specific factors (organ function, ability to swallow, etc.) and tolerability. Since many patients with mucositis experience painful swallowing, other formulations (such as IV or transdermal) often are necessary and should be considered.
Gabapentin
Another alternative for the treatment of mucositis pain in patients with head and neck cancer who are undergoing concomitant chemotherapy and radiation is gabapentin. This anticonvulsant has long been used for the treatment of postherpetic neuralgia and neuropathic pain.18 Although data are limited and additional research is needed, gabapentin may be considered as a treatment option for these patients.19
Conclusion
Mucositis is a common and painful side effect of treatment for many cancer patients. Although preventive strategies and good oral hygiene are key, the focus of treatment should be palliation of pain or discomfort. A stepwise approach is suggested based on NCCN findings. This strategy may include bland oral rinses, topical anesthetics, and systemic analgesics. As with all treatment, individual patient factors should be considered in the selection of appropriate pain-management strategies.
REFERENCES
1. Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120:1453-1461.
2. Rosen LS, Abdi E, Davis ID, et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J Clin Oncol. 2006;24:5194-5200.
3. Vagliano L, Feraut C, Gobetto G, et al. Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT—results of a multicentre study. Bone Marrow Transplant. 2011;46:727-732.
4. Bensinger W, Schubert M, Ang KK, et al. NCCN Task Force Report. Prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6(suppl 1):S1-S21.
5. Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820-831.
6. Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(suppl 9):1995-2025.
7. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. NIH Publication No. 09-5410. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed February 16, 2016.
8. Lilleby K, Garcia P, Gooley T, et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37:1031-1035.
9. Svanberg A, Birgegård G, Ohrn K. Oral cryotherapy reduces mucositis and opioid use after myeloablative therapy—a randomized controlled trial. Support Care Cancer. 2007;15:1155-1161.
10. Kepivance (palifermin) product information. Stockholm, Sweden: Swedish Orphan Biovitrum AB; March 2015.
11. Le QT, Kim HE, Schneider CJ, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808-2814.
12. Xylocaine Viscous 2% (lidocaine hydrochloride) product information. Lake Zurich, IL: Fresenius Kalbi USA, LLC; September 2014.
13. Cerchietti LC, Navigante AH, Bonomi MR, et al. Effect of topical morphine for mucositis-associated pain following concomitant chemoradiotherapy for head and neck carcinoma. Cancer. 2002;95:2230-2236.
14. Cerchietti LC, Navigante AH, Körte MW, et al. Potential utility of the peripheral analgesic properties of morphine in stomatitis-related pain: a pilot study. Pain. 2003;105:265-273.
15. Vayne-Bossert P, Escher M, de Vautibault CG, et al. Effect of topical morphine (mouthwash) on oral pain due to chemotherapy- and/or radiotherapy-induced mucositis: a randomized double-blinded study. J Palliat Med. 2010;13:125-128.
16. Sarvizadeh M, Hemati S, Meidani M, et al. Morphine mouthwash for the management of oral mucositis in patients with head and neck cancer. Adv Biomed Res. 2015;4:44.
17. Leenstra JL, Miller RC, Qin R, et al. Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: a phase III, randomized, double-blind trial (NCCTG-N09C6 [Alliance]). J Clin Oncol. 2014;32:1571-1577.
18. Neurontin (gabapentin) product information. New York, NY: Pfizer Inc; September 2015.
19. Bar Ad V, Weinstein G, Dutta PR, et al. Gabapentin for the treatment of pain syndrome related to radiation-induced mucositis in patients with head and neck cancer treated with concurrent chemoradiotherapy. Cancer. 2010;116:4206-4213.
To comment on this article, contact rdavidson@uspharmacist.com.





