US Pharm. 2023;48(4):HS2-HS5.
ABSTRACT: Vancomycin is a widely available antibiotic with gram-positive activity against Staphylococcus aureus, including methicillin-resistant S aureus (MRSA). In 2020, updated practice guidelines recommended a change in vancomycin therapeutic drug monitoring (TDM) from trough-based to AUC to minimum inhibitory concentration (AUC/MIC) ratio–based to optimize vancomycin efficacy and reduce nephrotoxicity. The goal AUC/MIC ratio recommended is 400 to 600 mg*hr/L. Bayesian software programs are the preferred method of vancomycin AUC monitoring by the guidelines; however, first-order pharmacokinetic equations can be used as an alternative. As healthcare institutions transition to AUC/MIC-based monitoring, pharmacists play a vital role in providing education on the guideline updates, implementation, and TDM.
Vancomycin is a glycopeptide antibiotic that is indicated in the management of gram-positive bacterial infections such as Streptococci, Enterococci, methicillin-susceptible Staphylococcus aureus (MSSA), and methicillin-resistant S aureus (MRSA). Vancomycin is bactericidal by inhibiting the polymerization of peptidoglycans in the bacterial cell wall and binding to the D-alanyl-D-alanine portion of the cell wall precursor. Weakening of the bacterial cell wall results in leaking of intracellular components and ultimately causes cell death. Vancomycin’s spectrum of activity makes it efficacious for infections such as bacteremia, skin and soft tissue infections, bacterial meningitis, osteomyelitis, and endocarditis caused by gram-positive bacteria, especially MRSA.1 Vancomycin displays AUC to minimum inhibitory concentration (AUC/MIC) ratio killing activity, meaning that overall drug exposure is important in optimizing its efficacy. Dosing recommendations are dependent on multiple factors, such as indication, patient weight, and renal function. Common adverse events associated with IV vancomycin include nephrotoxicity and infusion reactions. Vancomycin infusion reactions are characterized by pruritus and erythematous rash, typically involving the face, neck, or upper torso, and are caused by rapid infusion of the medication. These reactions are not considered allergies and can be prevented by slowing the vancomycin infusion rate to a maximum of 500 mg/30 min.2 To prevent other adverse reactions and to optimize efficacy, therapeutic drug monitoring (TDM) for vancomycin ensures that a safe and effective dose has been selected.1 Supratherapeutic dosing can result in adverse effects, such as nephrotoxicity, while subtherapeutic dosing can negatively impact patient outcomes if an infection is not properly treated.
Vancomycin can be administered intravenously, orally, or rectally depending on the indication. Both oral and rectal vancomycin are indicated for Clostridioides difficile infection, whereas IV vancomycin is indicated for various systemic infections. When administered orally, it is poorly absorbed, with a bioavailability of less than 10%. Rectal absorption through inflamed colonic mucosa is significant. IV vancomycin onset of action is rapid, with serum peak concentration achieved immediately after completion of infusion, while oral vancomycin onset of action is unknown. This medication has a large volume of distribution in body tissue and fluids ranging from 0.4 L/kg to 1 L/kg but not in cerebrospinal fluid. Protein binding is approximately 55%. There is no apparent metabolism, so it is excreted unchanged. Vancomycin has a biphasic elimination half-life. Terminal half-life for adults is 4 to 6 hours with normal renal function, but half-life is prolonged in renal impairment. Excretion occurs primarily via glomerular filtration, with IV vancomycin excreted via urine as 75% unchanged drug in 24 hours. Oral vancomycin is excreted in feces. In adults with normal BMI and renal function, clearance ranges from 1.6 to 6.2 L/hr.1,3
In 2009, an expert panel from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists published practice guidelines for vancomycin therapeutic monitoring for treatment of S aureus. It was recommended that trough serum vancomycin concentrations could be used as a surrogate marker for AUC/MIC monitoring, as trough levels provided an accurate and practical method for monitoring. Trough serum concentrations were recommended to be obtained at steady state just before the fourth dose. To prevent resistance, trough serum concentrations should be maintained at >10 mg/L. For complicated infections such as bacteremia, endocarditis, and hospital-acquired pneumonia, trough concentrations of 15 to 20 mg/L were recommended.4 In 2020, a revised consensus guideline for vancomycin TDM was released for patients with serious S aureus infections. The panel recommended a change in vancomycin TDM from trough-based to AUC/MIC-based, targeting levels of 400 to 600 mg*hr/L, assuming an MIC of 1 mg/L, to improve efficacy and minimize toxicity.5
Therapeutic Drug Monitoring
The guideline-recommended change in TDM to daily AUC/MIC ratio values between 400 to 600 mg*hr/L for patients receiving vancomycin was made in the setting of studies showing that acute kidney injury (AKI) rates are lower with AUC/MIC ratio versus traditional trough monitoring. Vancomycin AKI is defined as an increase in the serum creatinine level of >0.5 mg/dL or a 50% increase from baseline in consecutive daily readings, or a decrease in calculated creatinine clearance of 50% from baseline on 2 consecutive days in the absence of an alternative explanation.5 In the past, calculation of AUC was considered cumbersome, requiring collection of multiple serum concentrations within the same dosing interval and use of the linear-trapezoidal method to calculate AUC.5 However, this is no longer the case, as AUC can now be accurately estimated with limited sampling through Bayesian methods or using a peak and trough level with simple first-order pharmacokinetic equations.6
Bayesian methods for vancomycin dosing and monitoring are considered the preferred method by the guidelines and are based on Bayes’ theorem, which makes predictions about an individual’s vancomycin pharmacokinetics based on the way the drug behaved in a prior group of patients.5 Bayesian dose optimization software uses well-developed vancomycin population pharmacokinetic models and patient-specific vancomycin levels to calculate the optimal vancomycin dose for a patient.7 Utilizing Bayesian software for vancomycin dosing and monitoring has several advantages. First, initial vancomycin serum levels for dosing and monitoring can be collected within 24 to 48 hours and do not require the stringent sampling times that were needed when obtaining vancomycin trough levels. Additionally, Bayesian software can account for physiological changes, such as alterations to the patient’s renal function, which allows for more precise dosing recommendations. While Bayesian software can make pharmacokinetic estimates based on a single serum concentration, studies have demonstrated that utilizing two serum concentrations results in more accurate AUC predictions.8
Several Bayesian software programs are available for use in the United States, including DoseMeRx, InsightRx, and PrecisePK, which can be purchased by healthcare institutions. These programs offer key features such as opportunities for integration into electronic medical records, customer and technical support, and modeling for special patient populations.9-11 Other free Bayesian dosing software is available through websites such as ClinCalc.com and GlobalRxPH.com; however, these free calculators may not be appropriate for all patient populations.12,13 Careful consideration of program features should be considered when selecting which program is best for a specific institution. Despite widespread availability of Bayesian software programs, many institutions have not yet implemented AUC-based dosing due to barriers such as lack of familiarity, cost, and need for additional education.14
As an alternative to Bayesian methods, first-order pharmacokinetic calculations can be used to estimate AUC using a steady-state peak and trough vancomycin level, ideally within the same dosing interval.5 The first-order equations used to estimate AUC can be found in FIGURE 1.15-17 An advantage of using first-order equations to calculate AUC is that they are simple and less expensive to implement, and many clinicians are already familiar with the equations, making them easy to implement into practice; however, compared with Bayesian methods, first-order equations are unable to account for dynamic changes to patient physiology and represent only a snapshot of AUC for the sampling period.5
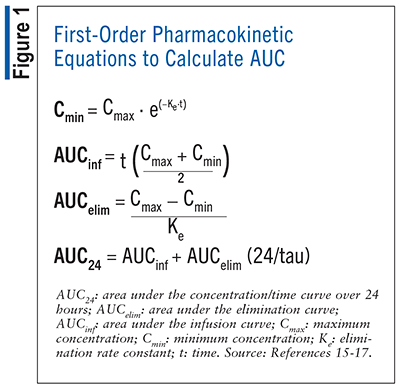
Recommendations on the timing of achievement of AUC/MIC ratio of 400 to 600 mg*hr/L have not been well established. Recent studies have shown a link between early achievement of AUC/MIC ratio and outcomes.18 Therefore, the guidelines recommend achievement within the first 24 to 48 hours of therapy.5
Administration
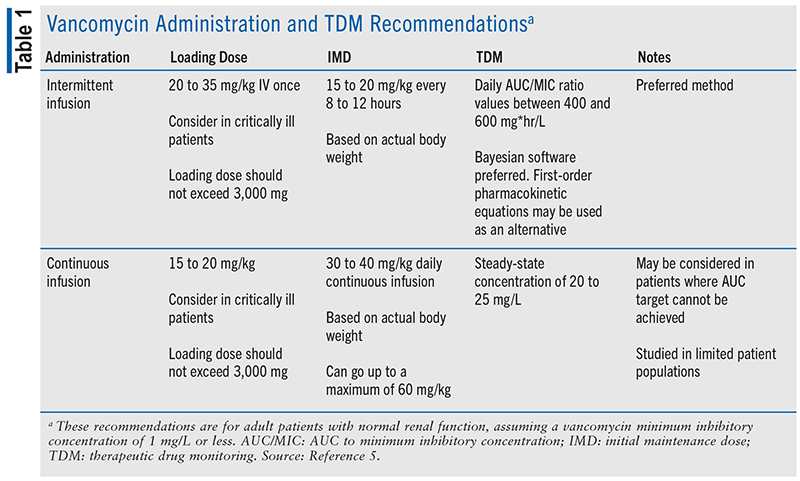
The recommended dosage of vancomycin is 15 to 20 mg/kg, based on actual body weight, administered every 8 to 12 hours as an intermittent infusion, in patients with normal renal function, assuming a vancomycin MIC of 1 mg/L or less. In order to achieve therapeutic concentrations more rapidly, a vancomycin loading dose of 20 to 35 mg/kg should be considered in critically ill patients prior to initiation of intermittent infusion. The loading dose should also be based on actual body weight and should not exceed 3,000 mg.5
As an alternative to intermittent infusion, continuous infusion has been studied in certain patient populations, specifically critically ill patients and patients receiving outpatient antimicrobial therapy. Potential benefits of continuous infusion vancomycin include less variation in serum levels, resulting in easier TDM, and possible lowering of the potential risk of AKI.19,20 Based on current guideline recommendations, continuous infusion vancomycin may be considered as an alternative to traditional intermittent infusion vancomycin when the AUC target cannot be achieved. A loading dose of 15 to 20 mg/kg, followed by a daily continuous infusion of 30 to 40 mg/kg to achieve a target steady-state concentration of 20 to 25 mg/L, can be considered in critically ill patients.5 A summary of vancomycin administration and TDM recommendations can be found in TABLE 1.
Considerations in Special Populations
The vancomycin TDM guidelines identify obese patients, pediatrics, and patients with renal impairment—including those on renal replacement therapy—as special populations. These patients display unique pharmacokinetic parameters that make dosing and monitoring more challenging. Specific recommendations for vancomycin dosing and therapeutic monitoring in these patient populations can be found within the vancomycin TDM guidelines.5
Role of the Pharmacist
As medication experts with extensive knowledge of pharmacokinetics, pharmacists are uniquely poised to aid in the transition from trough-based TDM to AUC/MIC ratio–based TDM. Pharmacists can provide education to all healthcare professionals on the 2020 vancomycin guideline updates through in-services and academic detailing. They are able to identify and recommend potential Bayesian software programs that would best align with an institution’s specific patient population and assist with adoption and implementation of the new guideline recommendations. Pharmacists may also be involved in creating policies and protocols surrounding vancomycin use, TDM, and conducting reviews to assess adherence to these protocols.
Additionally, if cost barriers prohibit implementation of software in electronic medical records, pharmacists can assist with the needed pharmacokinetic calculations or develop institution-specific dosing calculators to help with initial dosing recommendations. As pharmacists continue to monitor patients who are prescribed vancomycin, they can subsequently recommend appropriate dosing adjustments to achieve therapeutic goals while mitigating toxicities and adverse events. Previous studies have demonstrated that pharmacist involvement in vancomycin TDM improves efficacy and reduces toxicity.21,22 A recent meta-analysis including nearly 20,000 participants found that pharmacist involvement in vancomycin TDM significantly reduces rates of AKI, improves rates of attaining vancomycin target concentrations, and reduces the risk of mortality—demonstrating the essential role of pharmacists in vancomycin TDM.21
Conclusion
The updated vancomycin guideline recommendations to monitor AUC/MIC ratios were a significant change from the previously suggested trough-based monitoring. Healthcare providers may not feel comfortable with the pharmacokinetic calculations now needed for vancomycin TDM, or they may not be well versed in Bayesian software selection to help with obtaining AUC/MIC ratios. Pharmacists can play a key role in the successful implementation of the new recommendations and ensure that patients are dosed appropriately when being treated with vancomycin to prevent adverse reactions if supra-therapeutic drug concentrations are reached.
REFERENCES
1. Patel S, Preuss CV, Bernice F. Vancomycin. September 1, 2022. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022 Jan-. www.ncbi.nlm.nih.gov/books/NBK459263/. Accessed October 1, 2022.2. Martel TJ, Jamil RT, King KC. Vancomycin flushing syndrome. October 25, 2022. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022 Jan-. www.ncbi.nlm.nih.gov/books/NBK482506/. Accessed March 6, 2023.3. Vancomycin. In: Lexi-Drugs. Lexi-Comp, Inc. January 27, 2023. https://online-lexi-com.jerome.stjohns.edu/lco/action/doc/retrieve/docid/patch_f/7856?cesid=aWfsGrBMwnh&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dvancomycin%26t%3Dname%26acs%3Dtrue%26acq%3Dvancom#. Accessed January 29, 2023.
4. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325-327.5. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Amer J Health-Syst Pharm. 2020;77(11):835-864.
6. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve–based vancomycin dosing. Am J Health-Syst Pharm. 2018;75(24):1986-1995.7. Bayard DS, Jelliffe RW. A Bayesian approach to tracking patients having changing pharmacokinetic parameters. J Pharmacokinet Pharmacodyn. 2004;31(1):75-107.
8. Olney KB, Wallace KL, Mynatt RP, et al. Comparison of Bayesian-derived and first-order analytic equations for calculation of vancomycin area under the curve. Pharmacotherapy. 2022;42(4):284-291.9. DoseMeRx. Precision dosing: a smarter way to dose. 2020. https://doseme-rx.com/. Accessed January 10, 2023.
10. InsightRX. Precision dosing done right. 2021. www.insight-rx.com/#about. Accessed January 10, 2023.11. PrecisePK. Maximize clinical efficacy with PrecisePK. 2021. https://precisepk.com/how-it-works. Accessed January 10, 2023
12. ClinCalc.com. Vancomycin calculator. https://clincalc.com/Vancomycin/. Accessed January 10, 2023. 13. GlobalRPH. Vancomycin dosing: Bayesian analysis. https://globalrph.com/medcalcs/vancomycin-dosing-bayesian-analysis/. Accessed January 10, 2023.
14. Bradley N, Lee Y, Sadeia M. Assessment of the implementation of AUC dosing and monitoring practices with vancomycin at hospitals across the United States. J Pharm Pract. 2022;35(6):864-869.15. Pai MP, Neely M, Rodvold KA, et al. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57.
16. Sawchuk RJ, Zaske DE. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976;4(2):183-195.17. Pai MP, Russo A, Novelli A, et al. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162-3167.
18. Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycin exposure and failure rates among adult hospitalized patients with methicillin-resistant Staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545.19. Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother. 2001;45(9):2460-2467.
20. Ingram PR, Lye DC, Fisher DA, et al. Nephrotoxicity of continuous versus intermittent infusion of vancomycin in outpatient parenteral antimicrobial therapy. Int J Antimicrob Agents. 2009;34(6):570-574.21. Kunming P, Xiaotian J, Qing X, et al. Impact of pharmacist intervention in reducing vancomycin-associated acute kidney injury: a systematic review and meta-analysis. Br J Clin Pharmacol. 2023;89(2):526-535.
22. Joseph K, Ramireddy K, Madison G, et al. Outcomes of a pharmacist-driven vancomycin monitoring initiative in a community hospital. J Clin Pharm Ther. 2021;46(4):1103-1108.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.






