US Pharm. 2018;43(8):HS2-HS6.
ABSTRACT: An increase in oropharyngeal squamous cell carcinoma (OPSCC)—the eighth most common cancer in men—is occurring even though smoking and alcohol-consumption rates are on the decline. This increase has been attributed to human papillomavirus (HPV), particularly HPV16. The changing etiology of OPSCC calls for a reevaluation of diagnostic, preventive, and treatment guidelines for OPSCC. HPV-positive OPSCC has a better overall outcome than HPV-negative OPSCC, a fact that is reflected in the National Comprehensive Cancer Network’s 2018 clinical practice guidelines on OPSCC. New therapy regimens include de-escalation of therapy, transoral robotic surgery, and the possibility of using HPV vaccination to prevent HPV-positive OPSCC.
Oropharyngeal squamous cell carcinoma (OPSCC) is the eighth most common cancer in men.1 Despite improved understanding of the disease and advances in therapeutic interventions, OPSCC continues to be diagnosed at an advanced stage and the survival rate remains poor.1,2 It is estimated that 37,180 new cases of OPSCC in males and 14,380 in females will occur in 2018; OPSCC is also expected to result in 7,280 deaths in males and 2,750 in females.3
OPSCC is typically associated with tobacco and alcohol use.4 However, even with declining smoking rates, an increase in OPSCC has been noted, particularly in nonsmoking, middle-aged white men.4,5 The increase has been attributed to human papillomavirus (HPV), particularly HPV16.5 The changing etiology of OPSCC demands a reevaluation of diagnostic, preventive, and treatment guidelines for OPSCC. HPV-positive OPSCC is a subset of OPSCC that differs from HPV-negative OPSCC in its epidemiological, clinical, and molecular characteristics.5 These tumors have a better prognosis than HPV-negative tumors and vary in their clinical response.6-8
Risk Factors for OPSCC
As noted previously, the main risk factors for HPV-negative OPSCC are tobacco and alcohol consumption. A specific risk factor for HPV-related OPSCC is sexual behavior; this includes oral sex and sex with multiple partners.9
Diagnosis
Sometimes OPSCC is asymptomatic, but in other cases patients may present with a lump in the neck or throat; persistent sore throat; difficulty swallowing, opening the mouth fully, or moving the tongue; ear pain; or unexplained weight loss.10 A patient who has experienced any of these symptoms for more than 2 weeks should be referred to a physician.
Because HPV cannot be cultured from clinical specimens in the laboratory, diagnosis relies on cytologic, histologic, and molecular methods, including DNA, RNA, and protein-based tests.11,12 Diagnosis may be confirmed through imaging tests such as CT scan, MRI, and positron emission technology, and a barium swallow may be ordered to determine whether the cancer has spread to the esophagus.
Treatment
Standard treatment is dependent on the disease stage and on patient and clinician preferences. A multidisciplinary approach is necessary for successful outcomes, with each main treatment modality (surgery, radiotherapy, and chemotherapy/biological agents) being evaluated for each individual case.12,13
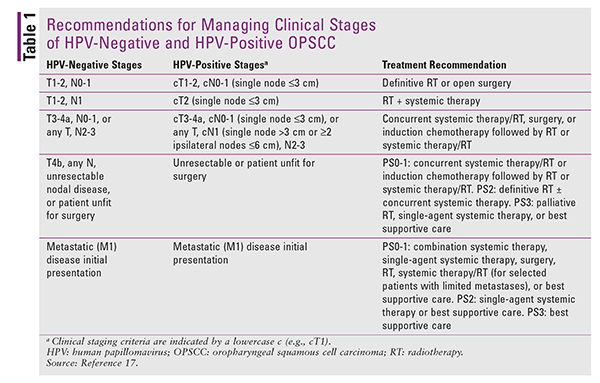
The management of OPSCC has changed since the emergence of HPV-positive OPSCC, which has better prognosis and survival rates. Therefore, the establishment of HPV status is a useful starting point for these therapies. The National Comprehensive Cancer Network’s (NCCN) 2018 tumor, node, metastasis classification for oropharyngeal cancer identifies available treatment options based on whether the tumor is HPV-positive or HPV-negative.17
TABLE 1 outlines the NCCN’s recommendations for managing the different clinical stages of HPV-positive and HPV-negative tumors. It is important to note that in HPV-positive OPSCC, pathological staging criteria differ from clinical staging criteria. Early-stage disease is generally managed with single-modality treatment, whereas advanced-stage disease requires chemoradiotherapy with or without neck dissection or surgical resection with reconstruction and postoperative chemoradiotherapy.13 Up-front radiotherapy is preferred because it is associated with comparable locoregional control and survival but has lower rates of severe complications.12 The median survival of patients with incurable or metastatic disease is 5 to 8 months, and research indicates that HPV-positive OPSCC may have longer survival rates that HPV-negative OPSCC.14-16
Concurrent cisplatin and radiotherapy are preferred in patients with locally advanced disease. After induction chemotherapy has been administered, radiation-based options include radiotherapy alone and radiotherapy with weekly carboplatin or cetuximab.17 Other chemotherapy agents include 5-fluorouracil, paclitaxel, docetaxel, and hydroxyurea; methotrexate, bleomycin, and capecitabine are used, but are less common.18 TABLE 2 lists the pharmacologic action, sample dosing schedule, and adverse effects of the commonly used chemotherapeutic agents. Pharmacists directly involved in chemotherapy preparation must carefully follow dosing guidelines and ensure the safety of all persons handling these preparations.19-21
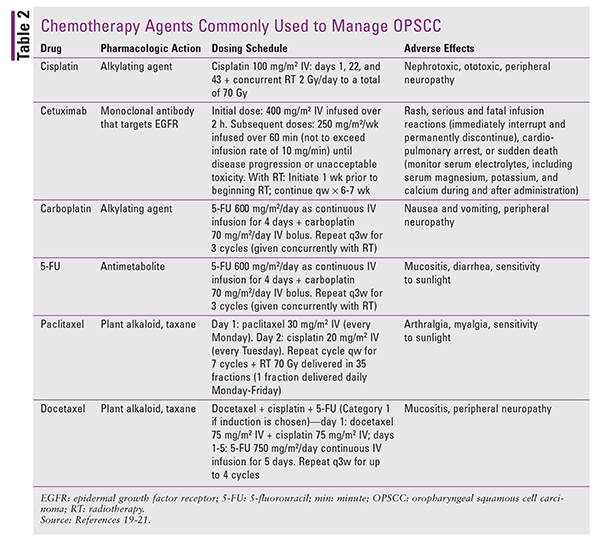
In addition to the drug-specific side effects mentioned in TABLE 2, more general adverse effects are observed with chemotherapeutic agents, including mouth sores, loss of appetite, nausea and vomiting, diarrhea, increased risk of infections due to low immunity, bruising and bleeding, and fatigue.22
Additionally, patients may experience late and long-term effects related to treatment. These include oral pain, skin changes, xerostomia (dry mouth), dental problems, trismus (lockjaw), changes in taste, excessive production of mucus, shoulder dysfunction, voice changes or hoarseness, and dysphagia (difficulty swallowing).22 Dysphagia may be self-limiting; however, it can become a chronic problem that can lead to nutritional problems, dehydration, and aspiration pneumonia. Dysphagia can be limited by regular exercises during and after therapy.22
Xerostomia can be caused by radiotherapy and is related to the amount of radiotherapy received. This condition can lead to increased dental caries, dysphagia, nutritional problems, and a disturbed sleep cycle. Pharmacists can review medication charts to ensure that patients are not taking any other medications, such as diuretics, that can aggravate xerostomia. Patients should be advised to follow a strict oral-hygiene routine, use a humidifier (especially in the bedroom), and carry a bottle of water at all times. Pharmacists may consider recommending that patients use commercial salivary substitutes, chew sugar-free gum, or suck on sugar-free candy.22
Future Therapies
Based on the increased response, better prognosis, and greater survival of patients with HPV-positive OPSCC, various management approaches are being explored, including de-escalation of therapy, the use of transoral robotic surgery (TORS), and HPV vaccination. The following four strategies for therapy de-escalation are being investigated.22
Radiation Combined With Cetuximab Instead of Cisplatin: Cetuximab is a monoclonal antibody that targets epidermal growth factor receptor, which is involved in the activation of several oncogenic pathways and is overexpressed in OPSCC. One study found that the addition of cetuximab to radiation provides better outcomes than radiation alone, and patients with early-stage disease seem to derive the greatest benefit from cetuximab.23 However, data on replacing cisplatin with cetuximab are conflicting, and a trial (RTOG 1016) is being conducted to reach a conclusion on this.24 Panitumumab and zalutumumab have been tested as alternatives to cetuximab; however, they are not currently used in the management of OPSCC.25
Induction Chemotherapy Followed by Reduced Radiation Doses and/or Volumes in Good Responders: Because response to chemotherapy predicts future response to subsequent radiotherapy, and HPV-positive OPSCC is presumably more radiosensitive that HPV-negative OPSCC, the option of induction chemotherapy followed by reduced doses of radiation is being explored by a number of trials, including ECOG 1308.22,26,27
Chemoradiation With Decreased Doses of Radiation and Chemotherapy: In one study, a deintensified chemotherapy regimen in patients with low-risk, HPV-positive OPSCC demonstrated a favorable pathological complete response.28 The regimen consisted of 60 Gy intensity-modulated radiotherapy (IMRT) instead of 70 Gy, with concurrent 30 mg/m2 cisplatin weekly instead of 100 mg/m2 on days 1, 22, and 43. Although this study had promising results for de-escalation of therapy, it is important to note that it was conducted over a very short period and in a very small patient cohort; therefore, further investigation is required before this schedule can be recommended.22
Other studies have shown that, in patients with HPV-positive OPSCC, the addition of chemotherapy to radiotherapy does not seem to significantly increase the overall survival benefit. One study showed good 3-year actuarial rates of overall survival, local-regional control, and distant metastasis-free survival when HPV-positive OPSCC patients received radiation only; however, the patient cohort was very small, so further investigation is required.29
Some studies have focused on using a different type of radiation in an attempt to reduce side effects and improve long-term functional outcomes. One trial is currently being conducted to compare proton therapy versus IMRT.30
TORS Followed, or Not Followed, by Postoperative Radiotherapy: TORS, a recently introduced procedure, enables resection of the pharyngeal tumor through the open mouth without the cosmetic deformity, morbidity, and functional deficits commonly associated with open surgery.22 It has been FDA-approved for the management of T1 and T2 tumors, and even though results are in the early phase, outcomes have been promising.22 Because this approach allows for more appropriate use of postoperative adjuvant therapy based on pathologic staging, patients may not need high-dose radiotherapy or concurrent chemoradiotherapy.22 Trials being conducted to further investigate the effects of TORS include ECOG 3311, which is investigating the efficacy of TORS followed by either low-dose or standard-dose radiotherapy or chemoradiotherapy; PATHOS, which is assessing whether swallowing function can be improved following TORS for HPV-positive OPSCC by reducing the intensity of adjuvant treatment protocols; and ADEPT, which is examining the intensity of adjuvant therapy required in p16-positive OPSCC patients.31-33
HPV Vaccination
FDA-approved vaccines for HPV-induced cervical and anal cancers have shown evidence of effectiveness in preventing HPV-positive OPSCC. One study demonstrated that HPV vaccination significantly reduced the prevalence of oral HPV16/18/6/11 in men (0.0% in vaccinated men vs. 2.13% in unvaccinated men).34 However, this trial had a number of limitations, and further research is required to establish this effect. Accordingly, HPV vaccines are not yet FDA-approved for this indication. Currently, three HPV vaccines are on the market: Gardasil, which protects against HPV types 6, 11, 16, and 18; Gardasil 9, which protects against types 16, 18, 31, 33, 45, 52, and 58; and Cervarix, which protects against types 16 and 18.35-37
Conclusion
The rising prevalence of HPV-positive OPSCC and the decline of HPV-negative OPSCC has brought OPSCC into the research limelight. Pharmacists need to not only provide patients with support and information on current therapies, but also stay abreast of emerging therapies that promise better outcomes for patients. An awareness of OPSCC is necessary for the timely referral and diagnosis of patients who may have this type of cancer. Pharmacists, therefore, must consider the possibility of OPSCC when patients present with symptoms that are suggestive of it.
REFERENCES
1. Cancer.Net. Oral and oropharyngeal cancer: statistics. www.cancer.net/cancer-types/oral-and-oropharyngeal-cancer/statistics. Accessed June 12, 2018.
2. Huber MA, Tantiwongkosi B. Oral and oropharyngeal cancer. Med Clin North Am. 2014;98(6):1299-1321.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Martín-Hernán F, Sánchez-Hernández JG, Cano J, et al. Oral cancer, HPV infection and evidence of sexual transmission. Med Oral Patol Oral Cir Bucal. 2013;18(3):e439-e44.
5. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50(5):380-386.
6. Yom SS. HPV and oropharyngeal cancer: etiology and prognostic importance. Semin Cutan Med Surg. 2015;34:178-181.
7. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261-269.
8. Dayyani F, Etzel CJ, Liu M, et al. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010;2:15.
9. Ryndock EJ, Meyers C. A risk for non-sexual transmission of human papillomavirus? Expert Rev Anti Infect Ther. 2014;12(10):1165-1170.
10. PubMed Health. Oropharyngeal cancer treatment (adult)(PDQ®). Health professional version. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032617/. Accessed June 12, 2018.
11. Burd EM, Dean CL. Human papillomavirus. Microbiol Spectr. 2016 Aug;4(4).
12. Urban D, Corry J, Rischin D. What is the best treatment for patients with human papillomavirus-positive and -negative oropharyngeal cancer? Cancer. 2014;120(10):1462-1470.
13. Elrefaey S, Massaro MA, Chiocca S, et al. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299-309.
14. Gibson MK, Li Y, Murphy B, et al; Eastern Cooperative Oncology Group. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(15):3562-3567.
15. Vermorken JB, Psyrri A, Mesía R, et al. OP041: impact of human papillomavirus (HPV) and p16 status on survival and response with cisplatin plus 5-FU and cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): analysis of the phase III extreme trial. Oral Oncol. 2013;49(suppl 1): S19-S20.
16. Vermorken JB, Stöhlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697-710.
17. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN® guidelines): head and neck cancers. Version 2.2018. www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed July 11, 2018.
18. American Cancer Society. Oral cavity and oropharyngeal cancer. www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer.html. Accessed February 8, 2018.
19. Flavill E, Fang YV, Miles B, et al. Induction chemotherapy followed by concurrent chemoradiotherapy for advanced stage oropharyngeal squamous cell carcinoma with HPV and P16 testing. Ann Otol Rhinol Laryngol. 2014;123(5):365-373.
20. Cancer Therapy Advisor. Head and neck cancers treatment regimens. www.cancertherapyadvisor.com/head-and-neck-cancer/head-neck-cancers-treatment-regimens/article/218124/. Accessed June 12, 2018.
21. Erbitux (cetuximab) package insert. Indianapolis, IN: Eli Lilly & Co; June 2018.
22. Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2017;8:4-11.
23. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578.
24. ClinicalTrials.gov. Radiation therapy with cisplatin or cetuximab in treating patients with oropharyngeal cancer. https://clinicaltrials.gov/ct2/show/NCT01302834. Accessed June 12, 2018.
25. Egloff AM, Grandis JR. Targeting epidermal growth factor receptor and SRC pathways in head and neck cancer. Semin Oncol. 2008;35:286-297.
26. Marur S, Li S, Cmelak AJ, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx—ECOG-ACRIN cancer research group. J Clin Oncol. 2017;35(5):490-497.
27. Chen AM, Felix C, Wang PC. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 2017;18:803-811.
28. Chera BS, Amdur RJ, Tepper J. Phase 2 trial of de-intensified chemoradiation therapy for favorable-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:976-985.
29. Chen AM, Zahra T, Daly ME, et al. Definitive radiation therapy without chemotherapy for human papillomavirus-positive head and neck cancer. Head Neck. 2013;35:1652-1656.
30. ClinicalTrials.gov. Randomized trial of intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for the treatment of oropharyngeal cancer of the head and neck. https://clinicaltrials.gov/ct2/show/NCT01893307. Accessed June 13, 2018.
31. ClinicalTrials.gov. Transoral surgery followed by low-dose or standard-dose radiation therapy with or without chemotherapy in treating patients with HPV positive stage III-IVA oropharyngeal cancer. https://clinicaltrials.gov/ct2/show/NCT01898494. Accessed June 13, 2018.
32. ClinicalTrials.gov. Post-operative adjuvant treatment for HPV-positive tumours (PATHOS). https://clinicaltrials.gov/ct2/show/NCT02215265. Accessed June 13, 2018.
33. ClinicalTrials.gov. Post operative adjuvant therapy de-intensification trial for human papillomavirus-related, p16+ oropharynx cancer (ADEPT). https://clinicaltrials.gov/ct2/show/NCT01687413. Accessed June 13, 2018.
34. Gillison ML, Broutian T, Graubard B, et al. Impact of HPV vaccination on oral HPV infections among young adults in the U.S. [abstract]. https://meetinglibrary.asco.org/record/153036/abstract. Accessed June 13, 2018.
35. Gardasil (human papillomavirus quadrivalent [types 6, 11, 16, and 18] vaccine, recombinant) package insert. Whitehouse Station, NJ: Merck & Co, Inc; April 2015.
36. Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant) package insert. Whitehouse Station, NJ: Merck & Co, Inc; February 2018.
37. Cervarix (human papillomavirus bivalent [types 16 and 18] vaccine, recombinant) package insert. Research Triangle Park, NC: GlaxoSmithKline; May 2016.
To comment on this article, contact rdavidson@uspharmacist.com.






