US
Pharm. 2006;1:20-24.
Osteoarthritis (OA) is the
most common joint disorder in the United States, affecting approximately 20
million Americans and accounting for significant disability and health care
expenditures in the U.S.1-5 Known as degenerative joint disease, OA
has been the second most common diagnosis, after chronic heart disease,
leading to Social Security disability payments due to long-term absence from
work.6 Principally affecting the elderly, OA is the leading cause
of disability in individuals older than 65 years and affects 70% to 90% of
those older than 75 years.7-9
The most common form of
arthritis, OA is a debilitating, degenerative disease of the articular
cartilage and synovial fluid.8 OA primarily affects the spine and
joints of the hand and lower extremities, resulting in pain, stiffness,
deformity, and loss of function.7,9,10 In elderly patients, some of
the most common chronic pain management cases involve OA, as well as low back
pain and neuropathy.5 While prevalence of the disease is equal
among both men and women, older women are twice as likely as men to have OA of
the knees and hands.11 The prevalence and severity of the disease
increases with age.11 Other risk factors for OA include mechanical
stress, joint injury, and obesity. Diagnosis is based on a physical exam,
comprehensive history, and radiographic findings.
While 85% of persons exhibit
evidence of OA after age 70, more than 50% of the population will have
radiographic evidence of the disease in at least one joint by age 65.5
Not all patients with radiographic evidence of OA have pain or functional
limitations.12 The best established predictor of disability in
patients with OA is muscle weakness, especially in patients with OA of the
knee.12 In fact, reduced quadriceps strength is an early finding in
subjects with knee OA; however, it is not clear whether reduced quadriceps
strength is a cause or a consequence of knee OA.13
Goals of Therapy
Goals of therapy
include controlling pain, improving and/or preserving joint function and
mobility, and improving health-related quality of life.1 While not
curable, established and experimental therapies seek to modify and/or even
reverse the course of OA.8 In order to improve functionality and
quality of life as a goal of pain management, a multidisciplinary application
of both nonpharmacologic and pharmacologic approaches tailored to the
individual is often required to provide the most effective therapeutic
outcomes.11,14-16 Communication between patients, clinicians, and
pharmacists is an important factor in the pain management process; the best
therapeutic outcomes may be obtained through an alliance among these
individuals.17
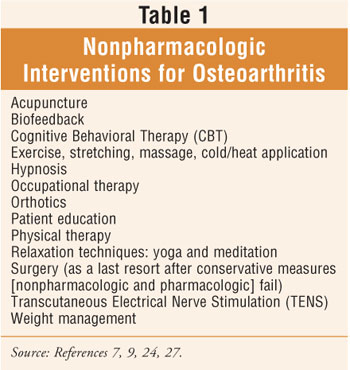
Nonpharmacologic Treatment
Nonpharmacologic
therapy, the foundation of the pharmaceutical care plan for OA, should be
utilized in all patients and started prior to or simultaneously with the
initiation of simple analgesics such as acetaminophen (up to 4 g/day) (table 1
).7,11
Exercise:
Regular exercise (e.g., strengthening, range of motion, isometric, isotonic,
isokinetic, postural) maintains healthy cartilage, encourages motility, and
helps develop muscles and tendons to absorb stress and prevent further damage
from OA.10,18,19 While stretching exercises are of particular
importance, inactivity or immobilization for even relatively short periods of
time may worsen or accelerate the clinical course of OA.19
Balancing exercise with adequate daytime rest (every four to six hours) allows
cartilage to rehydrate and is key. 19
A combination of aerobic
exercise (e.g., walking), resistance training (e.g., weight training), and
stretching (e.g., yoga) may be helpful for patients with OA. The warmth and
buoyancy of exercising in warm water (83°F to 88°F) may also assist with
discomfort and stiffness during movement.10 In some cases, a
well-planned exercise regimen has the ability to arrest OA of the hip and knee.
19
Physical Therapy:
For patients with physical disabilities due to OA, a standardized physical
therapy program can provide mobility benefits.5 Physical therapy
may even be beneficial in older nursing home residents up to age 89, as
behavior modification has been shown to reduce the need for pain medication,
the incidence of pain-associated behaviors (e.g., avoiding activities or
exercise, complaining of pain), and subjective reports of pain.5,20
Studies continue to confirm that the application of manual physical therapy
and supervised exercise produce greater symptomatic relief and functional
benefits in patients with OA of the knee.21,22
Surgery:
When conservative nonpharmacologic and pharmacologic therapy fails to relieve
pain or improve joint function, total joint replacement (total arthroplasty)
is highly effective for treating OA of the hip and knee.19 While
types of artificial joints and surgical techniques vary depending upon the
joints affected, replacing a joint almost always improves a patient's range of
motion and function and dramatically decreases pain.10 The needs
and capabilities of a potential surgical candidate should be clearly defined,
while considering the treatment goals of improved physical function and pain
relief.
Pharmacologic Intervention
Some experts
believe that drug therapy is the least important aspect of optimal disease
management, perhaps 15% of a total program.19 Whether drug therapy
or a nonpharmacologic approach is used, careful consideration of geriatric
pharmacokinetics and pharmacodynamics is necessary.5 Understanding
and comparing available therapies for OA can help individualize treatment,
which may include combination therapy.
In most patients with OA,
acetaminophen in increasing doses of up to 1 g four times daily (not to exceed
4 g/day) is the first choice for pain relief because the drug is effective and
generally safer than NSAIDs.7,11,19,23 For patients who show signs
of inflammation or do not respond to acetaminophen, treatment with an NSAID
should be considered. While the adverse effects (e.g., gastrointestinal [GI]
toxicity) of older NSAIDs (e.g., piroxicam, oxaprozin, naproxen, ibuprofen)
are similar, incidence varies by agent and individual patients. Seniors are at
high risk for adverse effects of NSAIDs; as many as 60% of seniors can develop
peptic ulceration and/or hemorrhage asymptomatically.23 The lowest
effective dose of an NSAID for the shortest possible duration is recommended
in this population. Declining renal function should also be considered,
especially when the creatinine clearance is 30 mL/min or less.23
Due to a decline in renal blood flow and glomerular filtration rate associated
with the progression of age, seniors may be particularly sensitive to
nephrotoxicity associated with NSAID therapy, especially if treatment consists
of concomitant NSAID and angiotensin II converting enzyme inhibitor (ACEI)
therapy.24 Elderly patients may demonstrate adverse effects from
NSAIDs at lower doses than younger adults. The long-term use of full-dosage,
longer half-life, non-COX-selective NSAIDs (e.g., naproxen, oxaprozin,
piroxicam) have the potential to produce GI bleeding, renal failure, high
blood pressure, and heart failure and are considered potentially inappropriate
medications in this population.23 Adverse effects to the central
nervous system include agitation, confusion, and hallucination.23
COX-2 inhibitors are used to
control inflammation and decrease pain. In April 2005, the FDA asked
manufacturers of all prescription nonselective NSAIDs and COX-2-selective
NSAIDs to revise their product labeling to include a boxed warning
highlighting the potential for increased cardiovascular events and/or any
well-described, serious, potentially life-threatening GI bleeding associated
with their use.25 Both older NSAIDs and COX-2 inhibitors can impair
renal function and cause sodium and water retention and should be used
cautiously in elderly patients, especially those with renal disease, volume
depletion, heart failure, or liver disease.7
Opioid analgesics can be used
to treat OA if they improve a patient's function and quality of life. However,
a patient's need for prolonged use of opioid analgesics should be evaluated to
avoid possible physical dependency.7 Tramadol, a synthetic opioid
agonist, has a rare side effect of seizures in those taking doses above the
recommended range, in patients with known seizure disorders, and in patients
receiving concomitant medications that lower the seizure threshold (e.g.,
antipsychotic agents, antidepressants).12 While corticosteroids
should not be used systemically, delivery as an intra-articular depot
injection is helpful when effusions (fluid accumulation that may cause
swelling) or signs of inflammation are present and should be limited to
infrequent and intermittent use. 10,19 They are indicated for the
symptomatic relief of pain from large joints unresponsive to usual therapy.
7
Intra-articular hyaluronic
acid injections have been shown to improve symptoms of knee OA; recent data
support their potential use as an effective long-term therapeutic option for
patients with knee OA.7,26 Topical preparations (e.g., capsaicin)
may be especially useful as monotherapy or as an adjunct to analgesic therapy
in patients with OA of the knees or hands.7,19 Oral nutraceuticals,
such as chondroitin and glucosamine sulfate, have shown promise for patients
with knee OA. Results of the NIH Glucosamine/Chondroitin Arthritis
Intervention Trial (GAIT) are expected to be released soon. The study is the
first multicenter trial in the
Researchers are seeking
established and experimental modalities to modify and/or reverse OA, which
include colchicine, bisphosphonates, hormones, dietary therapeutics such as
green tea and ginger, and experimental treatments such as growth factors, gene
therapy, matrix metalloproteinase inhibitors, nitric oxide, and cytokines.
8 Studies are exploring therapies that allow chondrocyte grafting or
preserve cartilage.19
Several factors, such as
impaired cognitive function, multiple potential causes of pain, unique
geriatric pharmacokinetics and pharmacodynamics, and clinician anxiety
regarding opioid addiction, may impede the assessment and pharmacologic
management of chronic pain in older individuals.5 Pharmacists in
various practice settings can have an active role by clarifying the
differences between agents, assisting in the assessment of pain (e.g., using
pain scales) on an ongoing basis, educating patients about the proper
administration of drugs, recommending appropriate agents, alternatives, and
adjustments in dosage, and monitoring for side effects and drug interactions
to assist with these important treatment issues.
Conclusion
Pharmacotherapy for
OA generally consists of analgesics, NSAIDs, COX-2 inhibitors,
corticosteroids, viscosupplementation, and symptomatic slow-acting drugs
(i.e., nutraceuticals). Improving function and quality of life are the goals
of pain management in elderly patients. To achieve these goals, a
multidisciplinary application of both nonpharmacologic and pharmacologic
approaches is often necessary. Both aerobic and strengthening exercises seem
to be equally effective to reduce pain and improve function. Management
regimens that can slow, alter, or reverse the degenerative process of OA
continue to be sought.
REFERENCES
1. Singh G.
Treatment options for osteoarthritis. Surg Technol Int.
2003;11:287-292.
2. Creamer P, Flores R,
Hochberg MC. Management of osteoarthritis in older adults. Clin Geriatr Med
. 1998;14:435-454.
3. Yelin E. The
economics of osteoarthritis. In: Brandt KD, Doherty M, Lohmander LS, eds.
Osteoarthritis.
4. Felson DT. The
course of osteoarthritis and factors that affect it. Rheum Dis Clin North Am
. 1993:19:607-615.
5. Freedman GM. Chronic
pain. Clinical management of common causes of geriatric pain. Geriatrics
. 2002;57:36-41.
6. National Istitiutes
of Health. Arthritis Prevalence Rising as Baby Boomers Grow Older:
Osteoarthritis Second Only to Chronic Heart Disease in Worksite Disability.
Available at: www.niams.nih.gov/ne/press/ 1998/05_05.htm. Accessed
7. Beers MH, Berkow R,
eds. The Merck Manual of Geriatrics. 3rd ed.
8. Fajardo M, Di Cesare
PE. Disease-modifying therapies for osteoarthritis: current status. Drugs
Aging. 2005;22:141-161.
9. Hinton R, Moody RL,
Davis AW, Thomas SF. Osteoarthritis: diagnosis and therapeutic considerations.
Am Fam Physician. 2002;65:841-848.
10. Beers MH, Jones TV,
Berkwits M, et al., eds. The Merck Manual of Health & Aging.
11. Boh LE, Elliott ME.
Osteoarthritis. In: DiPiro JT, Talbert RL, Yee GC, et al., eds.
Pharmacotherapy: A Pathophysiologic Approach. 5th ed.
12. Fraenkel L, Felson
D. Osteoarthritis. In: Hazzard WR, Blass JP, Halter JB, et al. Principles
of Geriatric Medicine and Gerontology. 5th ed.
13. Thorstensson CA,
Petersson IF, Jacobsson LT, et al. Reduced functional performance in the lower
extremity predicted radiographic knee osteoarthritis five years later. Ann
Rheum Dis. 2004;63:402-407.
14. Ferrell BA. The
management of pain in long-term care. Clin J Pain. 2004;20:240-243.
15. Horgas AL. Pain
management in elderly adults. J Infusion Nurs . 2003;26:161-165.
16. Fine PG.
Pharmacological management of persistent pain in older patients. Clin J Pain
. 2004;20:220-226.
17. Otis JAD, Fudin J.
Use of long-acting opioids for the management of chronic pain. US Pharmacist.
2005;March (suppl):3-12.
18. Bischoff HA, Roos
EM. Effectiveness and safety of strengthening, aerobic, and coordination
exercises for patients with osteoarthritis. Curr Opin Rheumatol.
2003;15:141-144.
19. Beers MH, Berkow R.
The Merck Manual of Diagnosis and Therapy. 17th ed.
20. Miller C, LeLieuvre
RB. A method to reduce chronic pain in elderly nursing home residents.
Gerontologist. 1982;22:314-317.
21. Deyle GD, Allison
SC, Matekel RL, et al. Physical therapy treatment effectiveness for
osteoarthritis of the knee: a randomized comparison of supervised clinical
exercise and manual therapy procedures versus a home exercise program. Phys
Ther. 2005;85:1301-1317.
22. Deyle GD,
23. Semla TP, Beizer
JL, Higbee MD. Geriatric Dosage Handbook. 10th ed.
24. Nolan TD, Abraham
PA, Matzke GR. Drug-Induced Renal Disease. In: DiPiro JT, Talbert RL, Yee GC,
eds, et al. Pharmacotherapy: A Pathophysiologic Approach. 5th ed.
25. Special Report.
COX-2 inhibitors: What does the future hold? Formulary .
2005;40:214-217.
26. Petrella RJ.
Hyaluronic acid for the treatment of knee osteoarthritis: long-term outcomes
from a naturalistic primary care experience. Am J Med Rehab.
2005;84:278-283.
27. National Initiative
on Pain Control. Multidisciplinary Pain Management in Long-Term Care.
Section 2: Nonpharmacologic and Pharmacologic Approaches to Pain. Thomson
Professional Postgraduate Services. 2005; ACPE program 381-999-05-027-H01:3-5.
To comment on this article,
contact
editor@uspharmacist.com.






