Diabetes mellitus is a group of metabolic disorders in which the body either does not produce enough insulin or is not able to utilize insulin. Two major types of diabetes mellitus are recognized. Type 1 diabetes, previously known as juvenile diabetes, is the result of the body’s failure to produce enough insulin. It is prevalent in 5% to 10% of the population with diabetes and is generally diagnosed in children.1 Type 2 diabetes is a result of insulin resistance (a condition in which the body cannot properly utilize insulin). This type of diabetes is more prevalent and is generally diagnosed in adults. Gestational diabetes affects approximately 2% to 7% of pregnant women.2 Incidences of diabetes are increasing at an alarming rate in epidemic proportions. According to the estimates of the World Health Organization, there are more than 180 million people with diabetes worldwide, and this number is likely to more than double by 2030.3
Osteoporosis is a condition characterized by low bone mass, increased fragility, and an increased fracture risk. According to the National Osteoporosis Foundation, approximately 10 million Americans are already suffering from the disease, and an estimated 34 million Americans have low bone mass.4 This accounts for 55% of the elderly population (aged 50 years and above).4 Women are more susceptible to osteoporosis than men. Several risk factors such as diet, smoking, and alcohol abuse are known to predispose individuals to osteoporosis. In addition, certain disease conditions such as anorexia, rheumatoid arthritis, gastrointestinal disorders, and diabetes mellitus are also considered risk factors for osteoporosis. In most patients, symptoms of osteoporosis are not observed until bone fracture. Older age presents greater risk for fractures that are associated with increased mortality.
Although traditionally osteoporosis has not been listed as a diabetes-related complication, increasing evidence suggests that patients with type 1 or type 2 diabetes are at an increased risk of osteoporotic fractures. This article will discuss the relationship between diabetes mellitus and osteoporosis.
Relationship Between Diabetes and Fractures
Osteoporosis in Type 1 Diabetes: It is well known that type 1 diabetes is associated with low bone mineral density (BMD) that may eventually lead to decreased bone mass in these patients during adolescence. This may explain higher incidences of osteoporotic fractures in postmenopausal women with type 1 diabetes. BMD is a measure of bone strength, which is routinely measured by dual-energy x-ray absorptiometry (DEXA). It was found that BMD in children with type 1 diabetes was 20% to 50% lower than in children without diabetes. One study examining vertebral BMD reported decreased cortical BMD in children with type 1 diabetes.5 Several studies in children and adults confirm that BMD is lower in patients with type 1 diabetes than in patients without diabetes.6-8 It is suggested that insufficient skeletal mineralization due to insulin deficiency may be responsible for decreased BMD in patients with type 1 diabetes.
There is also a strong link between type 1 diabetes and osteoporotic fractures. A large prospective cohort study of 32,089 postmenopausal women was conducted in Iowa. The study revealed that postmenopausal women who have diabetes or in whom diabetes develops are at higher risk for hip fracture than postmenopausal women without diabetes.9 Another survey, the Nord-Trøndelag Health Survey, showed a significant increase in hip fracture rates among females with type 1 diabetes compared to females without diabetes.10 Overall, current data clearly indicate that type 1 diabetes decreases BMD and increases risk for osteoporotic fractures.
Osteoporosis in Type 2 Diabetes: In contrast to the findings in patients with type 1 diabetes, BMD in type 2 patients has been found to be either normal or higher compared to those without diabetes. Studies in women with type 2 diabetes, controlling for age and obesity, show BMD that is either the same as or greater than that in normal subjects.11,12 Additional studies observed higher BMD levels in patients with type 2 diabetes treated with insulin, indicating that insulin deficiency may not be a determinant factor.6 A large cohort study was conducted in male patients with type 2 diabetes. This study found that the BMD of type 2 male patients was similar to that of men with normal glucose tolerance.13 Higher BMD is attributed to the higher obesity rate in the type 2 diabetes population. Previously, higher BMD in patients with type 2 diabetes was thought to be beneficial since higher BMD offers protection from osteoporosis. Ironically, in spite of higher BMD, most studies in women with type 2 diabetes have found that they are at double the risk of hip fractures compared to postmenopausal women without diabetes.9,10,14,15 Furthermore, the study of osteoporotic fractures in women older than 65 years with type 2 diabetes also found an increased risk of hip and proximal humerus fractures.16 There was also a trend toward increased risk of vertebral, forearm, ankle, and foot fractures.
In summary, even though BMD of patients with type 2 diabetes was found to be normal or higher, evidence is accumulating that these patients are at higher risk of osteoporotic fractures. One of the explanations for increased fractures in this population is a higher incidence of falls due to several diabetes-related complications such as neuropathy and retinopathy.
Osteoporosis in Gestational Diabetes: Gestational diabetes has not been reported to be associated with bone loss in prospective trials.17
Mechanism of Osteoporosis in Diabetes
The distinct observation that osteoporosis occurs in young patients with type 1 diabetes, even shortly after the onset of diabetes mellitus, has led to the hypothesis that insulin deficiency is a primary causative factor for osteoporosis. Insulin is an anabolic agent, deficiency of which leads to detrimental effects on the various properties of the bone such as bone strength, bone mineralized surface area, and osteoblast activity. It should be noted that in animal studies, insulin administration reverses all of these abnormalities and increases bone strength.18 In addition to insulin, osteoblasts also possess receptors for insulinlike growth factor-1 (IGF-1), a polypeptide similar to insulin. IGF-1 plays an important role in childhood growth. Serum levels of IGF-1 are found to be lower in patients with type 1 diabetes compared to patients without diabetes. Overall, these findings indicate that insulin deficiency may be responsible for increased osteoporotic fractures in patients with type 1 diabetes. But if this were the case, then patients with type 2 diabetes should not have a higher rate of osteoporotic fractures since they display only insulin resistance and not insulin deficiency. Therefore, it was proposed that hyperglycemia, a result of insulin deficiency or insulin resistance, is responsible for higher osteoporosis in the population with diabetes.
Higher glucose levels in the blood are known to interact with several proteins to form advanced glycation end (AGE) products. Yamagishi et al hypothesized that AGE-products in collagen may interact with bone to reduce bone strength, resulting in osteoporosis in patients with diabetes.19 Accumulated AGE-products in the body may stimulate apoptosis of osteoblasts, thereby contributing to deficient bone formation.20 AGE-products are specifically recognized by AGE receptors (RAGE). AGE-RAGE interaction is known to alter bone healing and bone turnover processes.21 Another indirect effect of hyperglycemia is glycosuria, which causes hypercalciuria, leading to decreased levels of calcium in the body and poor bone quality. Additionally, several diabetes-related complications such as retinopathy, neuropathy, and nephropathy have been linked to decreased BMD.22 Additional studies are required to determine the cause-and-effect relationship between specific mechanisms and increased risk of osteoporosis in patients with diabetes.
Treatment
Although there is no cure for osteoporosis, it can be treated. In the majority of cases, osteoporosis in diabetes is treated the same as osteoporosis in subjects without diabetes. Osteoporotic medications are mainly divided into two categories—antiresorptive medications and bone-forming (anabolic) medications (TABLE 1).
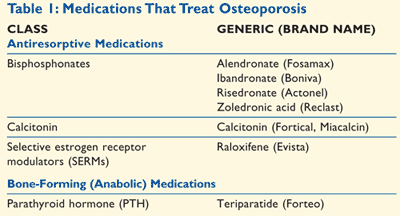
Antiresorptive Medications: Bisphosphonates such as alendronate, ibandronate, and risedronate are inhibitors of bone resorption that are clinically used to treat osteoporotic spine and hip fractures. Alendronate and risedronate have very poor absorption profile and therefore should be ingested on an empty stomach.17 Bisphosphonates are indicated for prevention as well as treatment of osteoporosis in postmenopausal women with type 2 diabetes.23 Bisphosphonates were also reported to reverse the deleterious actions of AGE-products on osteoblastic cells.24 Some reports suggest that diabetes mellitus may be a risk factor for bisphosphonate-related osteonecrosis and, therefore, that patients with diabetes mellitus being treated with bisphosphonates should be carefully monitored.25
It has been suggested that hormones such as estrogens and androgens can be used to treat osteoporosis. Since hormone replacement therapy (HRT) has been shown to increase BMD in the hip and spine, it was considered as a treatment choice. However, there are no data to support the hypothesis that increased BMD due to HRT indeed results in decreased incidences of fractures. Initially, the FDA had approved use of estrogen for the treatment of osteoporosis. However, now the FDA has removed this indication, and estrogen is approved only for the prevention of osteoporosis.17 In men, androgen replacement therapy has been shown to improve BMD.26 However, androgen therapy is contraindicated in patients with a history of prostate cancer.
Raloxifene, a selective estrogen receptor modulator, has been indicated in the prevention as well as treatment of osteoporosis.17 A 3-year, randomized, clinical trial demonstrated that vertebral fracture risk was reduced in postmenopausal women after raloxifene treatment.27 Additional randomized clinical trials confirmed that raloxifene does not affect insulin sensitivity or glycemic control in postmenopausal women with type 2 diabetes.28
The FDA has approved calcitonin for the treatment of osteoporosis in postmenopausal women.29 The physiologic importance of calcitonin is not clearly known. However, at the pharmacologic doses, calcitonin has been found to suppress osteoclast-mediated bone resorption. Inhibition of the resorption process decreases blood calcium levels and thereby helps to improve bone quality. One randomized clinical trial of calcitonin nasal spray in postmenopausal women with established osteoporosis observed that calcitonin prevents recurrence of osteoporotic fractures.30 During the development of synthetic calcitonins for therapeutic use in bone disease, Young et al observed a “diabetogenic” (hyperglycemic) effect with salmon calcitonin.31 This effect was attributed to inhibition of insulin secretion. Furthermore, Starke et al reported depressed circulating glucagon and glucose in insulin-dependent patients with diabetes after exogenous calcitonin administration.32 Treatment with calcitonin should be considered for older women with osteoporosis with painful vertebral fractures and for women who fail to respond to or cannot tolerate bisphosphonates. Calcitonin may also be indicated for women who are unable to take bisphosphonates because of impaired renal function.29
Bone-Forming Medications: Parathyroid hormone, a principal regulator of calcium homeostasis, is a potent anabolic agent for the treatment of osteoporosis. In November 2002, the FDA approved teriparatide, which is a synthetic form of human parathyroid hormone.33 Teriparatide is the first treatment that stimulates new bone growth and increases bone mass. This drug is well tolerated with minimal side effects. Once-daily administration of teriparatide resulted in increased BMD at the spine and proximal femur, and increased whole body BMD in men with osteoporosis.34 Postmenopausal women experienced dramatic reductions in the risk of vertebral and nonvertebral fractures when treated with teriparatide.35,36
Conclusion
It is very clear that patients with type 1 diabetes have decreased BMD and increased risk of fractures. Evidence is accumulating that even though patients with type 2 diabetes have higher BMD, they have an increased risk of osteoporotic fractures. Currently, treatment of osteoporosis in diabetes is the same as treatment in patients without diabetes. However, more clinical studies are required to study the efficacy and adverse effects of osteoporotic treatment in this population.
REFERENCES
2. Daniel KL, Borja NL. Gestational diabetes. US Pharm. 2007;32(10)(Diabetes suppl):17-20.
3. World Health Organization. Diabetes. Fact sheet No. 312. November 2008. www.who.int/mediacentre/
4. National Foundation of Osteoporosis. Fast facts on osteoporosis. July 2005. www.nof.org/awareness2/images/Fast_Facts.pdf. Accessed April 2, 2009.
5. Roe TF, Mora S, Costin G, et al. Vertebral bone density in insulin-dependent diabetic children. Metabolism. 1991;40:967-971.
6. Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196-1200.
7 . Valerio G, del Puente A, Esposito-del Puente A, et al. The lumbar bone mineral density is affected by long-term poor metabolic control in adolescents with type 1 diabetes mellitus. Horm Res. 2002;58:266-272.
8. Liu EY, Wactawski-Wende J, Donahue RP, et al. Does low bone mineral density start in post-teenage years in women with type 1 diabetes? Diabetes Care. 2003;26:2365-2369.
9. Nicodemus KK, Folsom AR; Iowa Women’s Health Study. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24:1192-1197.
10. Forsen L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and incidence of hip fracture: results from the Nord-Trøndelag Health Survey. Diabetologia. 1999;42:920-925.
11. van Daele PL, Stolk RP, Burger H, et al. Bone density in non-insulin-dependent diabetes mellitus: the Rotterdam Study. Ann Intern Med. 1995;122:409-414.
12. Hirano Y, Kishimoto H, Hagino H, Teshima R. The change of bone mineral density in secondary osteoporosis and vertebral fracture incidence. J Bone Miner Metab. 1999;17:119-124.
13. Barrett-Connor E, Holbrook TL. Sex differences in osteoporosis in older adults with non-insulin-dependent diabetes mellitus. JAMA. 1992;268:3333-3337.
14. Meyer HE, Tverdal A, Falch JA. Risk factors for hip fracture in middle-aged Norwegian women and men. Am J Epidemiol. 1993;137:1203-1211.
15. Paganini-Hill A, Ross RK, Gerkins VR, et al. Menopausal estrogen therapy and hip fractures. Ann Intern Med. 1981;95:28-31.
16. Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32-38.
17. Chau DL, Edelman SV. Osteoporosis and diabetes. Clin Diabetes. 2002;20:153-157.
18. Dixit PK, Ekstrom RA. Decreased breaking strength of diabetic rat bone and its improvement by insulin treatment. Calcif Tissue Int. 1980;32:195-199.
19. Yamagishi S, Nakamura K, Inoue H. Possible participation of advanced glycation end products in the pathogenesis of osteoporosis in diabetic patients. Med Hypotheses. 2005;65:1013-1015.
20. Alikhani M, Alikhani Z, Boyd C, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345-353.
21. Santana RB, Xu L, Chase HB, et al. A role for advanced glycation end products in diminished bone healing in type I diabetes. Diabetes. 2003;52:1502-1510.
22. Munoz-Torres M, Jodar E, Escobar-Jimenez F, et al. Bone mineral density measured by dual X-ray absorptiometry in Spanish patients with insulin-dependent diabetes mellitus. Calcif Tissue Int. 1996;58:316-319.
23. Ikeda T, Manabe H, Iwata K. Clinical significance of alendronate in postmenopausal type 2 diabetes mellitus. Diabetes Metab. 2004;30:355-358.
24. Gangoiti MV, Cortizo AM, Arnol V, et al. Opposing effects of bisphosphonates and advanced glycation end-products on osteoblastic cells. Eur J Pharmacol. 2008;600:140-147.
25. Khamaisi M, Regev E, Yarom N, et al. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. J Clin Endocrinol Metab. 2007;92:1172-1175.
26. Francis RM. Androgen replacement in aging men. Calcif Tissue Int. 2001;69:235-238.
27. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637-645.
28. Andersson B, Johannsson G, Holm G, et al. Raloxifene does not affect insulin sensitivity or glycemic control in postmenopausal women with type 2 diabetes mellitus: a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:122-128.
29. Silverman SL. Calcitonin. Endocrinol Metab Clin North Am. 2003;32:273-284.
30. Chesnut CH III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in post-menopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267-276.
31. Young AA, Wang MW, Gedulin B, et al. Diabetogenic effects of salmon calcitonin are attributable to amylin-like activity. Metabolism. 1995;44:1581-1589.
32. Starke A, Keck E, Berger M, Zimmermann H. Effects of calcium and calcitonin on circulating levels of glucagon and glucose in diabetes mellitus. Diabetologia. 1981;20:547-552.
33. Teriparatide information. www.teriparatide.org. Accessed April 13, 2009.
34. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
35. Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19:745-751.
36. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434-1441.
To comment on this article, contact rdavidson@jobson.com.





