US Pharm. 2009;34(7):39-41.
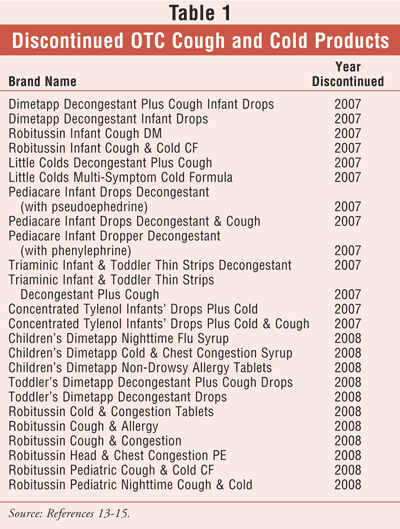
According to a recent survey of pharmacists, more than 60% of OTC recommendations sought by the public involved cough and cold products.1 This is no surprise, since the common cold is the most frequently occurring illness worldwide and the leading cause of missed school or work days.2 While giving advice about OTCs is one of the pharmacist's duties, it is getting increasingly hard to provide this service, particularly when it concerns children. In October 2007, the FDA held a Public Advisory Committee meeting to discuss and develop recommendations for the safe use of pediatric OTC cough and cold products.3 This meeting resulted in new labeling for some children's cough and cold products and spurred the removal of some formulations from the market.4 (See TABLE 1.) From the time this information began trickling into community pharmacies, pharmacists have been struggling to provide appropriate counseling regarding pediatric OTC cough and cold products.
FDA Recommendation
The October 2007 joint meeting of the FDA's Nonprescription Drugs and Pediatric advisory committees determined that there was no proof that OTC medications eased cold symptoms in children under 12 years of age.3 The committees did find, however, that rare reports of serious side effects and even deaths have been associated with pediatric use of OTC cough and cold preparations. This forum had been pursued by Baltimore Health Commissioner Dr. Joshua Sharfstein, who became an advocate for OTC cough and cold safety in children following the overdose-related deaths of four children in the Baltimore area.4,5
An FDA review of records from 1969 to 2006 revealed more than 120 pediatric deaths arising from overdose of antihistamines or decongestants.5,6 The Centers for Disease Control and Prevention (CDC) reported that, in emergency departments between 2004 and 2005, there were more than 1,500 cases of children under 2 years of age who experienced adverse drug effects or overdose associated with cough and cold products. Driven by these alarming numbers, the National Association of Medical Examiners and the CDC began searching for fatalities associated with cough and cold medications in children under 12 months of age. For 2005, these investigators found three conclusive deaths--all in infants under the age of 6 months--associated with OTC cough and cold preparations.7
Arizona has a Child Fatality Review Program under which every child mortality in that state is investigated. In 2006, a reported 90 unexpected child fatalities occurred in Arizona. Toxicology studies had been performed in only 21 cases of infant fatality. Ten of these cases were associated with recent administration of OTC cough and cold products. One of the infants had been dispensed a cough and cold medication in a baby bottle.6
In January 2008, the FDA--on recommendations from the CDC, the American Academy of Pediatrics, and the Consumer Healthcare Products Association--issued a public health advisory to caution against the use of OTC cough and cold products in children under 2 years of age.6 The advisory further stated that a review of OTC cough and cold medications for children aged 2 through 11 years is currently underway.8
Dosing Recommendations
Given that most--if not all--adverse events have been due to overdoses, a brief history of dosing recommendations for pediatric OTC products is in order. In 1972, the FDA commenced a large-scale review of the hundreds of OTC cough and cold formulas then on the market. An expert panel that included consumers was convened to prepare a statement on recommendations for OTC cough and cold products. The resulting statement, released in 1976, supported the use of OTC cough and cold products in adults, but cited a lack of data for marketing such products for children under 2 years of age. Dosages for children aged 6 through 11 years were unscientifically determined to be half of those for an adult; dosages for children aged 2 through 6 years were approximated to be a quarter-strength of adult dosages. The FDA permitted manufacturers to list a category on packages for children younger than 2 years of age if the recommendation directed the consumer to seek advice from a physician. Until the Public Advisory Committee meeting in October 2007, no review of these pediatric dosages was conducted by the FDA.4
Advice Concerning Pediatric OTC Products
The FDA's recommendations and manufacturers' withdrawal of products for children under 2 years of age have changed the way pharmacists advise caregivers. Today, OTC cough and cold products carry a "do not use" recommendation for children under 2 years of age.8 Some OTC products have extended this recommendation to children under 6 years of age. Given that the FDA has not completed its review of the use of OTC cough and cold products in children aged 2 through 11 years, products still carry labeling indicating that they can be administered to children older than 2 years of age.9 The FDA also recommended that manufacturers place a "do not use to sedate children" warning on pediatric OTC cough and cold products.8
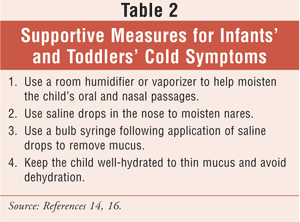
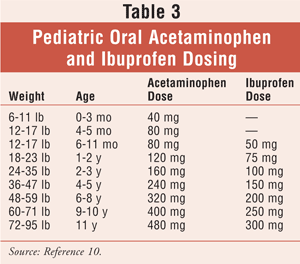
Despite all the changes and recommendations, supportive procedures for symptomatic relief do exist. Supportive measures that can be offered by a pharmacist are given in TABLE 2. It is important to note that antipyretics such as acetaminophen and ibuprofen have dosing recommendations for infants (TABLE 3). Although the common cold is a self-limiting disease, it can lead to changes in the nasal passageways and predispose a patient to secondary bacterial infections, including those of the respiratory tract.2,5 It is critical to identify and refer a seriously ill infant to an appropriate health care provider (HCP). A caregiver without a primary care provider can benefit from a prepared list of local HCPs. A list of clinics or charitable organizations that offer monetary assistance is useful for community pharmacies, which may encounter caregivers who seek a pharmacist's advice because they cannot afford a doctor's visit.
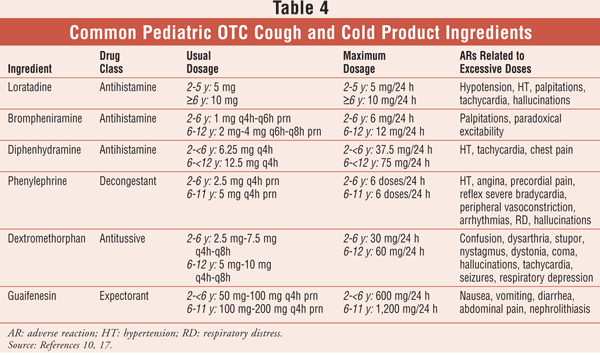
In addition to the supportive measures listed in TABLE 2, discussion of the use of accurate measuring devices should be part of every counseling session for pediatric OTCs. Caregivers should be educated against using eating utensils, baby bottles, or nongraduated bottle caps for dispensing medication. Each ingredient should be discussed in terms of its indication and possible side effects (TABLE 4).
One vital piece of information that is often overlooked in counseling, even for prescription medications, is the consideration of maximum dosage. Many resources are available to pharmacists seeking this information. The American Pharmacists Association carries an extensive selection of reference books and software on its Web site. The Lexi-Comp products are particularly user-friendly and are offered in various specialties, including pediatric drugs and geriatric drugs.
Maximum dosages for some pediatric cough and cold ingredients are found in TABLE 4. Careful adherence to maximum-dosage warnings includes taking into account all medications the patient is taking. For example, many OTC cough and cold products contain acetaminophen, and administration of additional acetaminophen could potentially surpass maximum dosage and possibly lead to hepatotoxicity.10
The most important aspect of the counseling session for pediatric OTCs is the taking of a complete history. This should include duration of present illness, underlying medical conditions, and a medication history (including OTCs, herbals, and folk remedies). Vital to the counseling process is the clinical evaluation. If the patient looks sick (e.g., listless, lethargic), referral to an HCP is in order. Caregivers of older children can be advised on a case-by-case basis, given a good knowledge of the child's history and utilization of reliable references.
To appropriately answer pediatric OTC-related questions, it is important to stay informed about new products and dosage recommendations. Regular perusal of the information found in pharmacy journals and other such sources, and the sharing of that information with colleagues, can certainly enhance a pharmacy practice. An up-to-date reference library not only is a legal requirement by state boards of pharmacy, it also is quite valuable for obtaining current guidelines and dosages.11
Conclusions
Pharmacists take an oath in which they pledge to "apply knowledge, experience, and skills to the best of [their] ability to assure optimal drug therapy outcomes for the patients" served. This oath states additionally that a pharmacist promises to "keep abreast of developments and maintain professional competency."12 That commitment to the public is made when the pharmacist is accepted into the profession. Communities throughout the nation rely on this commitment and on the dedication with which pharmacists execute their professional services.
The caregiver seeking a pediatric OTC cough and cold product for his or her infant may initially be disappointed to find that these products are no longer available. A brief account of the new FDA recommendations and the reasons behind these changes can help assure the caregiver of the pharmacist's concern for the patient's welfare. A key to success in counseling about pediatric OTCs is genuine empathy. Patients can sense sincere concern, and this in turn establishes the foundation for a good patient-pharmacist relationship.
In acquiring knowledge of the latest information and guidelines, the pharmacist not only builds up his or her own armamentarium, but also helps educate patients and caregivers seeking pharmacy counseling. The changing landscape of pediatric OTC cough and cold dosage recommendations has altered the dynamics of pharmacists' OTC advice to caregivers. Pharmacists need to continue to update and educate themselves regarding future changes in dosing recommendations for pediatric cough and cold OTC products.
REFERENCES
1. Levy S. Pharmacist knows best. OTC Recommendation Survey 2007. Drug Topics. 2007;151:36-40.
2. Doerr SE. Common cold.
www.medicinenet.com/common_
3. FDA. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Pediatric Advisory Committee. Agenda. October 18, 2007.
www.fda.gov/ohrms/dockets/ac/
4. Sharfstein J, North M, Serwint J. Over the counter but no longer under the radar--pediatric cough and cold medications. New Engl J Med. 2007;357:2321-2324.
5. Ryan T, Brewer M, Small L. Over-the-counter cough and cold medication use in young children. Pediatr Nurs. 2008;34:174-180,184.
6. Rimsza M, Newberry S. Unexpected infant deaths associated with use of cough and cold medications. Pediatrics. 2008;122:318-322.
7. Srinivasan A, Budnitz D, Shehab N, Cohen A. Infant deaths associated with cough and cold medications--two states, 2005. MMWR. 2007;56:1-4.
8. FDA. Public Health Advisory. Nonprescription cough and cold medicine use in children. August 15, 2007.
www.fda.gov/cder/drug/
9. Magill-Lewis J. Are OTC cold remedies safe for kids? Drug Topics. 2008;152:54.
10. Taketomo CK, Hodding JH, Kraus DM, eds. Pediatric Dosage Handbook. 15th ed. Hudson, OH: Lexi-Comp; 2008:42,903.
11. Texas Pharmacy Laws and Regulations. Charlottesville, VA: LexisNexis Matthew Bender; 2009:114.
12. American Association of Colleges of Pharmacy. Oath of a pharmacist.
www.aacp.org/resources/
13. Tylenolprofessional.com. Questions and answers: pediatric cough cold medicine voluntary withdrawal.
www.tylenolprofessional.com/
14. Woo T. Pharmacology of cough and cold medicines. J Pediatr Health Care. 2008;22:73-79.
15. Wyeth.com. Wyeth Consumer Healthcare initiates voluntary recall and replacement program for several Robitussin products and Children's Dimetapp Cold & Chest Congestion. October 29, 2007.
www.wyeth.com/news/archive?
16. Tylenol.com. Your child's cold.
http://tylenol.com/page.jhtml ?id=tylenol/children/
17. Jellin J. Dosing of OTC products in the pediatric population for healthcare professionals. Pharmacist's Letter. 2006;22:220107.
To comment on this article, contact rdavidson@jobson.com.






