US Pharm. 2022;47(11):24-26.
ABSTRACT: Type 1 diabetes mellitus (T1DM), an autoimmune disease, affects more than 1 million people in the United States, and approximately 50% of these patients will develop complications related to elevated blood glucose. Currently there are no FDA-approved therapies for delaying the onset or progression of T1DM; however, teplizumab—a monoclonal antibody that has shown efficacy in clinical trials—is being reviewed by the FDA. If it is approved, teplizumab will become the first disease-modifying therapy for T1DM. Because of their high accessibility to patients, pharmacists would be able to engage in early identification of those patients in whom the use of this agent would be most beneficial.
Diabetes, a chronic health condition that impairs how the body converts sugar into energy, affects more than 37 million people in the United States.1 There are three main types of diabetes: type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and gestational diabetes (GDM). T1DM occurs when the body’s insulin-producing cells (beta cells) are attacked by T cells via an autoimmune process. Insulin, a hormone produced by the body’s pancreatic beta cells, is required to convert glucose into energy, which is essential for survival. More specifically, insulin stimulates glucose transport across cell membranes via the translocation of glucose transporters to the plasma membrane; this process enables glucose to enter the cells of muscle, liver, and fat to be used for energy. Because they are unable to produce their own insulin for regulating blood-glucose levels, patients with T1DM must take multiple daily injections of exogenous insulin or receive a continuous infusion of insulin.
Formerly known as juvenile diabetes, T1DM is usually diagnosed in children and young adults, with the peak age being around 13 to 14 years.2 Damage to pancreatic beta cells begins months to years before symptom onset, so once symptoms manifest, they can be quite severe. The prevalence of T1DM in adults ranges from 5% to 10%.
By comparison, although T2DM also is caused by dysregulation of insulin in the body, the dysregulation is due to the body’s resistance to insulin, as opposed to the loss of insulin production in T1DM.2 In contrast to T1DM, T2DM usually develops over years and is more prevalent in adults than in children. The third main type of diabetes, GDM, can develop in pregnant women with no history of diabetes. The exact cause of GDM is unclear, but this condition is thought to be due to the major hormonal changes that take place during pregnancy.
Genetic and environmental factors contribute to the risk of developing T1DM and T2DM, and the risk of T1DM is higher in children with affected close relatives because of gene polymorphisms.2 If symptoms of hyperglycemia go unnoticed and high blood sugar remains untreated, a patient is at risk for life-threatening acute complications, such as diabetic ketoacidosis (DKA). Although anyone with diabetes is at risk for developing DKA, patients with T1DM—because of the absence of insulin production—are far more likely than those with T2DM to develop DKA; in fact, it is often the first sign in undiagnosed T1DM patients.3 Long-term complications of uncontrolled diabetes include damage to the body’s small and large blood vessels, potentially leading to heart attack, stroke, blindness, and kidney damage, and the risk of these complications increases the longer the diabetes remains untreated. Studies have shown that the best method for preventing acute and chronic complications is strict control of blood-glucose levels via medications and lifestyle modifications.4
Current Therapeutic Options for T1DM
The complete destruction of pancreatic beta cells, combined with a lack of appropriate islet-cell repair mechanisms, ultimately leads to impaired glycemic control in T1DM.5 Exogenous insulin replacement therapy is essential for patients with T1DM. The only FDA-approved option for adjunctive therapy in T1DM is pramlintide. Although they are not currently approved for clinical use in the U.S., whole pancreatic transplantation and pancreatic islet-cell transplantation are potential options for the treatment of T1DM.
Islet-cell transplantation faces many challenges. Because it is not a standard treatment for T1DM in the U.S. and is considered experimental, participating hospitals must request permission from the FDA to conduct clinical research. Additional difficulties include poor vascular engraftment after transplantation, extensive islet death after transplantation, a lack of available islets post transfer, and the need for matching donors. However, efforts are being made to harness stem cells’ benefits for islet transplantation to improve the efficacy.5
Pramlintide is an amylin analogue that works to complement insulin and regulate glucose in the bloodstream after meals by mimicking the action of the naturally occurring hormone amylin. Pramlintide’s three primary mechanisms of action are 1) slowing the emptying of the stomach, 2) suppressing secretion of the hormone glucagon after a meal, and 3) increasing satiety levels. Although clinical trials have demonstrated reductions in A1C and modest weight loss, pramlintide’s adverse effects and the necessity of additional injections have limited this agent’s clinical use.6,7
Whereas in patients with T2DM the decision to initiate insulin therapy is based on disease severity, insulin treatment is essential for T1DM patients immediately upon diagnosis because the hallmark of T1DM is absent or near-absent beta-cell function. Insulin replacement therapy regimens typically consist of basal (long-acting) insulin and bolus (rapid-acting) insulin.6 Basal insulin mimics the background insulin a healthy pancreas creates during the day, whereas bolus insulin manages rises in blood glucose (e.g., following a meal). Because of the pathophysiology of T1DM, it is imperative for these patients to be on a daily insulin regimen to prevent acute complications. However, it is important to note that although insulin therapy may delay the onset of complications by controlling hyperglycemia, it is not a cure for T1DM, nor does it slow the onset.
The American Diabetes Association (ADA) recommends that the intensity of pharmacologic treatment be individualized based on the patient’s A1C goals and ability to self-manage. Along with insulin therapy, the ADA guidelines also recommend the adoption of lifestyle modifications (e.g., exercise) along with medical and nutritional therapy.6
Introduction to Teplizumab
Although current options for delaying the onset or progression of T1DM may seem limited, there is cause for optimism. Teplizumab, a humanized anti-CD3 (cluster of differentiation 3) monoclonal antibody, is currently being evaluated for its ability to prevent T1DM, and the data look promising. Its mechanism of action is not fully understood, but as illustrated in FIGURE 1, teplizumab reduces the autoimmune destruction of pancreatic beta cells by modifying CD8+ T lymphocytes—effector cells that kill beta cells—via decreased activation, signaling, and gene cell expression.8 This is thought to result in favorable outcomes in T1DM, at least in part, via partial or transient exhaustion of CD8 effector T cells.9
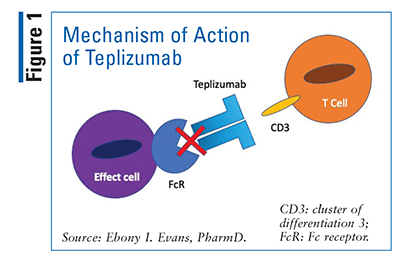
Given the high prevalence of T1DM and the limited number of options for treating it or delaying progression, many studies have been conducted to search for alternatives, including a number of trials involving teplizumab. Previous studies were able to demonstrate that teplizumab prolonged the production of insulin in T1DM.8 However, the Teplizumab Prevention Study was the first to test teplizumab’s ability to delay or prevent disease progression in children and adults at high risk for developing T1DM. Given the risk of long-term complications in T1DM, delaying disease progression can have far-reaching benefits for patients’ longevity and quality of life.
Evidence for Teplizumab
Herold and colleagues conducted a phase II, randomized, placebo-controlled, double-blind trial of teplizumab involving relatives of patients with T1DM who were at high risk for development of clinical disease (i.e., relatives of individuals with T1DM who had two or more autoantibodies and abnormal blood-sugar levels).10 Seventy-six patients were randomly assigned to treatment with a single 14-day course of teplizumab (n = 44) or placebo (n = 32). Treatment was found to delay the time to diagnosis of T1DM: Fewer patients in the teplizumab group were diagnosed with T1DM (43% vs. 72%), and the median time to diagnosis was longer in the teplizumab group (48.4 months vs. 24.4 months). The benefits of teplizumab were shown to be greatest within the first 3 years after administration, with a median 2-year delay in diagnosis of T1DM. Teplizumab treatment was associated with a decrease in lymphocyte count, which returned to normal by day 15; there was no resultant difference in infection rates between the two treatment groups.10
In an extended follow-up analysis of participants in the abovementioned trial, Sims and colleagues found an increased and prolonged benefit from teplizumab.11 This study showed an ongoing delay of diabetes with teplizumab, with a median time to diagnosis of roughly 5 years (vs. 2.3 years in the placebo group) as well as improved rates of insulin production.11 Both Herold and Sims plan to continue follow-up every 6 months, in patients who continue to consent to trial inclusion, in the hope of seeing sustained disease delay over a longer period of time.
Sims and colleagues conducted another study to determine whether metabolic end points detected an effect of teplizumab on beta-cell decline within 3 months of treatment in high-risk patients.12 Using glucose and C-peptide response curves, the researchers demonstrated that teplizumab delays rapid metabolic decline and improves the metabolic state within 3 months.12
With regard to currently ongoing research, ClinicalTrials.gov lists a phase III trial, PROTECT (PROvention T1D Trial Evaluating C-peptide with Teplizumab), which is investigating the efficacy and safety of teplizumab in patients with newly diagnosed T1DM.13
Despite promising data showing that treatment with teplizumab delayed the diagnosis of clinical T1DM in high-risk study participants, this agent is not yet available for use in patients, as it is still undergoing the approval process. The FDA has extended its review of the Biologics License Application for teplizumab, and the new PDUFA (Prescription Drug User Fee Act) date is November 17, 2022.14 If the FDA grants approval, teplizumab will become the first disease-modifying therapy for use in T1DM.
The Pharmacist’s Role
More than 1 million people in the U.S. have T1DM, and approximately 50% of them will develop a complication over the course of this disease.15,16 The pharmacist’s role in the management of T1DM, including slowing its progression, will continue to expand.
As of this writing teplizumab is still awaiting FDA approval; however, pharmacists should become familiar with available information on this agent. As medication experts, pharmacists should keep abreast of emerging treatments and the changing landscape of diabetes pharmacotherapy. Given that teplizumab has been shown to provide the most benefit in the first 3 years, early identification of patients would be of paramount importance. Pharmacists are the most accessible point of contact in the healthcare system for many patients, and they are well equipped to engage in the early identification of those patients for whom teplizumab therapy would be most beneficial.
Conclusion
The availability of a disease-modifying agent could lead to a major shift in the approach to T1DM management. If it receives FDA approval, teplizumab has the potential to positively impact the health outcomes and quality of life of many patients with T1DM. Early identification of those patients who could most benefit from the use of teplizumab would be critically important.
REFERENCES
1. CDC. National Diabetes Statistics Report. www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed August 18, 2022.
2. CDC. Diabetes basics. www.cdc.gov/diabetes/basics/index.html. Accessed August 18, 2022.
3. Cleveland Clinic. Diabetes-related ketoacidosis (DKA). https://my.clevelandclinic.org/health/diseases/21945-diabetic-ketoacidosis-dka. Accessed August 19, 2022.
4. Hess-Fischl A, Leontis LM. Type 2 diabetes complications: how to prevent short- and long-term complications. EndocrineWeb. www.endocrineweb.com/conditions/type-2-diabetes/type-2-diabetes-complications. Accessed August 19, 2022.
5. Pathak V, Pathak NM, O’Neill CL, et al. Therapies for type 1 diabetes: current scenario and future perspectives. Clin Med Insights Endocrinol Diabetes. 2019;12:1179551419844521.
6. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64(12):2609-2652.
7. Pullman J, Darsow T, Frias JP. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc Health Risk Manag. 2006;2(3):203-212.
8. Tooley JE, Vudattu N, Choi J, et al. Changes in T-cell subsets identify responders to FcR-nonbinding anti-CD3 mAb (teplizumab) in patients with type 1 diabetes. Eur J Immunol. 2016;46(1):230-241.
9. Long SA, Thorpe J, DeBerg HA, et al. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol. 2016;1(5):eaai7793.
10. Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603-613.
11. Sims EK, Bundy BN, Stier K, et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med. 2021;13(583):eabc8980.
12. Sims EK, Cuthbertson D, Herold KC, Sosenko JM. The deterrence of rapid metabolic decline within 3 months after teplizumab treatment in individuals at high risk for type 1 diabetes. Diabetes. 2021;70(12):2922-2931.
13. ClinicalTrials.gov. Recent-onset type 1 diabetes trial evaluating efficacy and safety of teplizumab (PROTECT). https://clinicaltrials.gov/ct2/show/NCT03875729. Accessed August 28, 2022.
14. Formulary Watch. FDA extends review of diabetes therapy teplizumab. www.formularywatch.com/view/fda-extends-review-of-diabetes-therapy-teplizumab. Accessed August 20, 2022.
15. Bullard KM, Cowie CC, Lessem SE, et al. Prevalence of diagnosed diabetes in adults by diabetes type—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):359-361.
16. Cleveland Clinic. Type 1 diabetes. https://my.clevelandclinic.org/health/diseases/21500-type-1-diabetes. Accessed August 27, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





