Since the introduction of the first proton pump inhibitor (PPI) in 1989, this class of medications has become a staple in the management of gastroesophageal reflux disease (GERD) and other acid-related disorders. PPIs are potent agents that significantly reduce acid secretion by irreversibly binding to H+/K+ adenosine triphosphatase, or the proton pump, located in the parietal cells. Overall, these medications have among the highest sales levels in the United States, approaching nearly $10 billion dollars in 2012.1
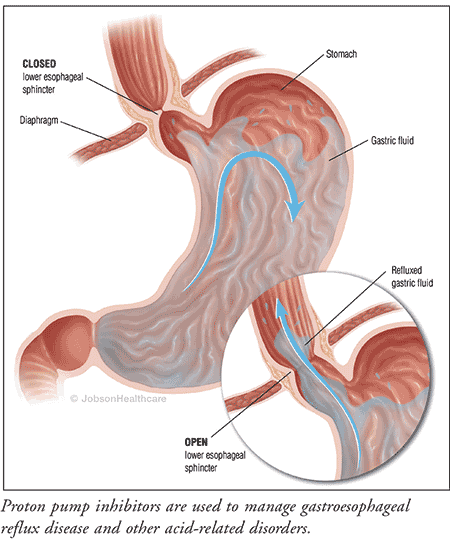
PPIs have proven to be very effective and safe in managing GERD, healing peptic ulcer disease, and reducing the incidence of nonsteroidal anti-inflammatory drug–associated gastropathy, becoming one of the most-prescribed medications by healthcare providers. Their superb efficacy and low toxicity resulted in the approval of the first OTC product in 2003, providing patients with an option other than antacids and H2-receptor antagonists for self-medication of ailments such as heartburn and other related symptomatology. These same factors have also contributed to their overuse and misuse; healthcare providers are often prescribing these agents for prolonged—even lifetime—use, and many patients are taking the OTC agents beyond the recommended course of therapy without any supervision.
Over the years, there has been a growing concern over potential adverse effects associated with long-term therapy. Some of these concerns include hypergastrinemia, development of pneumonia, dementia, and drug interactions.2-4 Since 2010, the FDA has issued various safety warnings regarding the potential effects of long-term use of PPIs: risk of fractures, hypomagnesemia, Clostridium difficile–associated diarrhea, vitamin B12 deficiency, acute interstitial nephritis (AIN), and cutaneous and systemic lupus erythematosus events.5 Pharmacists play an important role in ensuring the appropriate use of these medications (Table 1), emphasizing appropriate dosing and self-monitoring during counseling sessions. Pharmacists should monitor for potential adverse effects, especially with prolonged use. Potential drug interactions should be identified and minimized with both prescription and OTC medications.

Hypergastrinemia
Gastric acid suppression leads to hypergastrinemia. This condition causes rebound hyperacidity; after discontinuing PPI therapy, patients may experience worsening GERD symptoms. This course of therapy can be as short as 8 weeks.2,3 To avoid this, PPIs should be slowly tapered. In addition, hypergastrinemia can cause parietal cells to hypertrophy and enterochromaffin-like cells (ECL) to undergo hyperplasia.3,6 These effects may increase the risk for gastric cancer, but this relationship has mostly been observed in vitro; however, a case study has recently been published describing the first case of ECL cell–derived neuroendocrine carcinoma as a result of hypergastrinemia secondary to more than 15 years of PPI usage.2,7 Despite this, evidence does not support an increased risk of cancer in patients using PPIs.2,6,8
Pneumonia
PPI use has been associated with an increased risk of developing community-acquired pneumonia (CAP). Acid suppression leads to an increase in gastric pH, allowing for the overgrowth of non-Helicobacter pylori bacteria in gastric juices, gastric mucosa, and the duodenum.2 This can potentially lead to microaspiration and lung colonization. PPIs also impair immune-defense mechanisms. Current evidence, however, has not provided conclusive findings.2,9,10 Therapy should not be withheld from patients with pulmonary disease if indicated. It is important to ensure that patients who are at risk for CAP, including the immunocompromised, elderly, smokers, and those with COPD and asthma, receive their annual influenza and recommended pneumococcal vaccinations.
C difficile
Gastric acid is an important defense mechanism against pathogens colonizing the stomach and intestinal tract. Recently, there has been concern regarding an increased incidence of C difficile colitis in PPI users; several studies have indicated an increased risk of C difficile infections.2,4,11 This increase could be attributed to a higher gastric pH, which could lead to a more virulent strain of bacteria. The delay in gastric emptying can prolong exposure to the bacteria.2 PPIs should be used with caution in patients who are at an increased risk for C difficile, including the immunocompromised, the elderly, hospitalized patients, and those taking broad-spectrum antibiotics; consider an H2-receptor antagonist as an alternative.
Fractures
Findings from epidemiologic studies have suggested an increased risk in hip, spine, and wrist fractures in patients on high does and/or long- term therapy.12,13 A causal relationship has been noted between acid suppression and reduced absorption of mineral calcium in the diet; there may be as much as a 41% reduction in calcium absorption after 14 days of omeprazole therapy.2,14 Although findings from various studies have been inconsistent, there is enough evidence to prompt revision of PPI labeling to include information about “possible increased risk of fractures of the hip, wrist, and spine.” Due to the inconsistencies, osteoporosis prophylaxis or routine bone mineral density screening is not recommended in patients receiving long-term PPI therapy. Given the significant morbidity and mortality from fractures, especially hip fractures, risks and benefits should be considered prior to initiating treatment with high-dose and/or long-term therapy in vulnerable patient populations; the lowest effective dose and shortest duration of therapy should be used to minimize risk. When calcium supplementation is used in conjunction with PPI therapy, citrate formulations should be considered rather than carbonate to maximize bioavailability.
Hypomagnesemia
Although rare, hypomagnesemia is associated with PPI use and can be life-threatening. Symptoms include muscle weakness and cramps, tetany, convulsions, arrhythmias, and hypotension. Patients may also present with secondary hypocalcemia and hypokalemia.2,3 Since 2006 there have been fewer than 30 cases reported; however, PPI users appear to have a 40% higher risk of hypomagnesemia.15,16 Upon discontinuation, magnesium levels normalized within 1 to 2 weeks, but reoccurred within days after attempts to restart PPI therapy.2 Most cases were associated with a duration of therapy of 5 or more years.15 Baseline serum magnesium levels should be obtained prior to initiating long-term therapy and monitored periodically thereafter. Caution should be taken when coadministering with other agents that may lower magnesium levels, such as digoxin and diuretics.
Vitamin B12 Deficiency
There have been some data to suggest an association between long-term PPI use and vitamin B12 deficiency, especially in the elderly. Malabsorption of vitamin B12 may result from atrophic gastritis and achlorhydria, promoting bacterial overgrowth that allows for the increased digestion of cobalamin.3 Results from studies have been inconsistent and appear to not be clinically significant. Most patients who consume a normal diet probably will not experience any significant B12 deficiency.2 Routine screening of vitamin B12 may be considered in the elderly or malnourished patients receiving long-term therapy, since this patient population has a higher prevalence of B12 deficiency.2
Acute Interstitial Nephritis
There have been case reports that have associated PPI use with the development of AIN.4,11 This is a humoral- and cell-mediated hypersensitivity reaction that can occur within days of therapy initiation and as long as 18 months thereafter.4,11 Upon discontinuation of the PPI, most patients spontaneously recovered.4 Patients should be educated about the symptoms of AIN, including nausea, vomiting, fatigue, fever, and hematuria. The onset is usually insidious; PPI therapy should be discontinued if AIN develops.
Dementia
Recent data has suggested a link between PPI use and dementia.17-19 Biologically, PPIs may increase the production and degradation of amyloid and bind to tau. The possibility of reduced levels of vitamin B12 and other nutrients may also play a role in the increased risk of dementia.20 These observational studies suggest an association, but no causal relationship has been established.
Subacute Cutaneous Lupus Erythematosus
Drug-induced lupus erythematosus (DILE) is a lupus-like syndrome that usually resolves after discontinuation of the medication.21 Drug-induced subacute cutaneous lupus erythematosus (SCLE) is the most common form of DILE. This condition is characterized by annular and papulosquamous skin lesions, typically occurring on sun-exposed areas of the body, including the neck, back, shoulders, and upper extremities.21,22 There have been a number of cases reported of PPI-induced SCLE.21,23 Most patients demonstrated cutaneous involvement only, occurring anytime between 1 week and almost 4 years of PPI therapy.21,23 The resolution period after drug discontinuation was 3 months, on average, with no or minimal symptomatic therapy needed.21 Although rare, it is important to ensure that patients are aware of this possibility, especially those at high risk for developing SCLE, including women of childbearing age, those with drug allergies or previous episodes of SCLE, photosensitive skin, exposure to ultra-violet radiation, and family history.24
Drug Interactions
Several significant drug interactions are associated with PPIs due to their ability to dramatically reduce gastric acid production and raise gastric pH. Medications that require an acidic environment for absorption may have reduced oral bioavailability in patients treated with PPIs. Some examples of agents that may be affected and have reduced efficacy include, but are not limited to, itraconazole, ketoconazole, isoniazid, oral iron supplements, and several protease inhibitors. If alternative therapies cannot be used, patients receiving these medications should be counseled to take them towards the end of the PPI dosing interval and be monitored for appropriate responses to therapy.25
Several studies have suggested that PPIs may inhibit the hepatic cytochromes involved in the metabolism of certain medications, raising concerns of additional drug interactions. Recently, attention has focused on the potential of PPIs to inhibit CYP2C19 and adversely affect the prodrug clopidogrel from being metabolized to its active form. Theoretically, such an interaction could reduce clopidogrel’s antiplatelet effect and lead to increased risk of cardiovascular events.26,27 However, the clinical significance of this interaction has been questioned, as two randomized, controlled trials have demonstrated no observable adverse outcomes when PPIs have been used concomitantly with clopidogrel.28,29
Conclusion
PPIs have revolutionized the management of acid-related diseases. There is strong evidence supporting their superior efficacy and overall safety profile. Unfortunately, this has also led to their overuse and inappropriate use. When used appropriately, the overall benefits significantly outweigh the potential risks in most patients. Almost half of all patients taking a PPI do not have a clear indication.30 Nearly all adverse effects associated with PPIs occur among patients who receive long-term therapy. It is important to note, however, that most studies published have been observational in nature and do not necessarily suggest a causal relationship. Pharmacists are in an ideal position to ensure appropriate and effective use and reduce PPI overuse. Through effective counseling and provision of medication therapy management sessions, pharmacists can ensure that PPI use is associated with appropriate indications utilizing the lowest effective dose for the shortest duration possible.
PATIENT INFORMATION
Proton Pump Inhibitors
What are PPIs?
PPIs are a class of medications that help reduce the amount of acid that your stomach makes. Several common conditions for which they can be used include heartburn and the treatment and prevention of stomach ulcers.
What are the available PPIs? Do I need a prescription?
Currently, there are six PPIs available: dexlansoprazole (Dexilant), esomeprazole (Nexium), lansoprazole (Prevacid), omeprazole (Prilosec), pantoprazole (Protonix), and rabeprazole (Aciphex). All are available by prescription. There are also products available OTC in both brand and generic forms, including Prevacid 24h, Nexium 24h, Prilosec OTC, and Zegerid (a combination of a PPI with an antacid).
How should PPIs be taken?
You should be sure to read the label or product packaging and follow the directions for use. However, generally speaking, these products are taken by mouth once daily, 30 to 60 minutes before breakfast.
What side effects are associated with PPIs?
PPIs are generally well tolerated. The most common side effects reported include headache, diarrhea, nausea, and vomiting. Reports of more serious side effects include kidney disease, fractures, infections and vitamin deficiencies, but these are very rare and are generally associated with long-term use (using these products for more than a year).
Are PPIs safe to use with other medications?
PPIs may make some medications less effective by reducing their absorption from the stomach. Be certain to read the product packaging carefully and to consult with your pharmacist before using these agents with other prescription and OTC products, including vitamin and nutrition supplements.
How long should I take PPIs?
OTC products should not be used for more than 2 weeks unless you are told to do so by your healthcare provider. You should not abruptly discontinue the use of prescription products unless you are told to do so by your healthcare provider, since you may experience heartburn and other related stomach symptoms.
Remember, if you have questions, Consult Your Pharmacist.
REFERENCES
1. Consumers Union. Proton pump inhibitors (PPI) medicines review. Consumer Reports Best Buy Drugs. July 2013. www.consumerreports.org/cro/2013/07/best-drugs-to-treat-heartburn-and-gerd/index.htm. Accessed June 22, 2017.
2. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci N. Y. 2011;56(4):931-950.
3. Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Ther Adv Gastroenterol. 2012;5(4):219-232.
4. Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6(4):443-451.
5. Drugwatch.com. Proton pump inhibitors. https://www.drugwatch.com/proton-pump-inhibitors/. Accessed May 11, 2017.
6. Graham DY, Genta RM. Long term proton pump inhibitor use and gastrointestinal cancer. Curr Gastroenterol Rep. 2008;10(6):543-547.
7. Jianu CS, Lange OJ, Viset T, et al. Gastric neuroendocrine carcinoma after long-term use of proton pump inhibitor. Scand J Gastroenterol. 2012;47(1):64-67.
8. Schneider JL, Kolitsopoulos F, Corley DA. Risk of gastric cancer, gastrointestinal cancers and other cancers: a comparison of treatment with pantoprazole and other proton pump inhibitors. Aliment Pharmacol Ther. 2016;43(1):73-82.
9. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176(2):172-174.
10. Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther. 2010;31(11):1165-1177.
11. Johnson DA, Oldfield EC. Reported side effects and complications of long-term proton pump inhibitor use: dissecting the evidence. Clin Gastroenterol Hepatol. 2013;11(5):458-464.
12. Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170(9):765-771.
13. Yang Y-X, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953.
14. Chubineh S, Birk J. Proton pump inhibitors: the good, the bad, and the unwanted. South Med J. 2012;105(11):613-618.
15. Hess MW, Hoenderop JGJ, Bindels RJM, Drenth JPH. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405-413.
16. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241.
17. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
18. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
19. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Proton pump inhibitors and risk of dementia. Ann Transl Med. 2016;4(12):240.
20. Kuller LH. Do proton pump inhibitors increase the risk of dementia? JAMA Neurol. 2016;73(4):379-381.
21. Sandholdt LH, Laurinaviciene R, Bygum A. Proton pump inhibitor-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2014;170(2):342-351.
22. Callen JP. Drug-induced subacute cutaneous lupus erythematosus. Lupus. 2010;19(9):1107-1111.
23. Reich A, Maj J. Subacute cutaneous lupus erythematosus due to proton pump inhibitor intake: case report and literature review. Arch Med Sci AMS. 2012;8(4):743-747.
24. Aggarwal N. Drug-induced subacute cutaneous lupus erythematosus associated with proton pump inhibitors. Drugs—Real World Outcomes. 2016;3(2):145-154.
25. Wedemeyer R-S, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf. 2014;37(4):201-211.
26. Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180(7):713-718.
27. Li X-Q, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos Biol Fate Chem. 2004;32(8):821-827.
28. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374(9694):989-997.
29. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363(20):1909-1917.
30. Pasina L, Urru S a. M, Mandelli S, et al. Evidence-based and unlicensed indications for proton pump inhibitors and patients’ preferences for discontinuation: a pilot study in a sample of Italian community pharmacies. J Clin Pharm Ther. 2016;41(2):220-223.
To comment on this article, contact rdavidson@uspharmacist.com.






