US Pharm. 2013;38(3):HS8-HS11.
ABSTRACT: In hydrocephalus, a disease or anatomical defect causes an increase in the amount of cerebrospinal fluid (CSF) present in the cranium, which most commonly results in increased pressure against the brain tissue. Hydrocephalus is difficult to diagnose because of its varied signs and symptoms, which commonly overlap with those of other medical conditions. There are generally two approaches to the treatment of hydrocephalus: 1) shunt placement and 2) endoscopic third ventriculostomy (surgical creation of an opening in the floor of the third ventricle to enable the passage of CSF). The treatment of hydrocephalus often requires long-term care and lifelong follow-up, especially in children and neonates in whom there is a congenital cause.
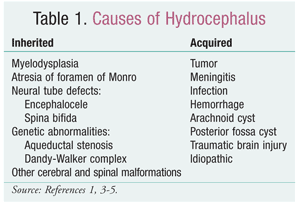
Hydrocephalus is a condition in which a disease state or defect causes an increase in the amount of cerebrospinal fluid (CSF) present in the cranium, most commonly resulting in increased pressure against the brain tissue.1 The word hydrocephalus translates to “water on the brain,” but the condition is caused by a variety of disorders and disease states, thus making it complex to define and diagnose.1 Most CSF is produced by the choroid plexus in the third and fourth ventricles in the brain.2 It fills the subarachnoid spaces, protecting and cushioning the brain.2 As more CSF is produced, it runs from the brain to the spinal cord and is removed from circulation via the arachnoid villi and the vertebral venous plexus.2 When hydrocephalus occurs, part of this process is blocked, although different parts are blocked depending upon the type of hydrocephalus. Causes range from obstructions to congenital diseases (TABLE 1).1,3-5
Classification
Because hydrocephalus has many different causes, it is difficult to classify specific varieties. As a result, many different classification systems have been developed. The most common way to classify hydrocephalus is to determine whether a case is 1) congenital or acquired or 2) obstructive or communicating. Congenital hydrocephalus is caused by a birth defect or genetic disorder; acquired hydrocephalus has other causes, such as a hemorrhage, infection, or tumor.1 Obstructive hydrocephalus is caused by an obstruction of CSF drainage by a tumor, congenital defect, or infection.6 Most cases of hydrocephalus are obstructive. Communicating hydrocephalus is caused by an overproduction of CSF that allows insufficient drainage time, or occurs when CSF is not absorbed at a normal rate. Examples include cranial hemorrhage and meningitis.6
Other classifications have been developed that specify the type of hydrocephalus, such as normal pressure hydrocephalus (NPH). A relatively new classification system is the multicategorical hydrocephalus classification (McHC), which is complicated and involves eight different categorical sections, including cause, pathophysiology, and occurrence of shunt placement.7 McHC is little known and seldom used, but it does provide a more accurate description of each patient’s case as it progresses. Regardless of the cause and subsequent classification, hydrocephalus patients present with similar symptoms (although these differ with age) and have the same limited number of treatment options.
Detection and Diagnosis
It is difficult to detect hydrocephalus because of its varied signs and symptoms, which commonly overlap with those of other diseases. Infants are likely to have a different disease progression from that of children and adults. Nausea and vomiting are common in infants and adults. In infants, fussiness and poor appetite are common indicators of hydrocephalus; however, the most prominent sign is a distended skull.1,3 The sutures in infant skulls are soft and not fully developed, which allows them to expand upon increased pressure from CSF accumulation.1,3 The increased intracranial pressure commonly causes headaches in adults and children, as their skull bones are not flexible. Difficulty with concentration and memory, a lack of balance, decreased bladder control, and a downward gaze are the most obvious signs in adults and children.1
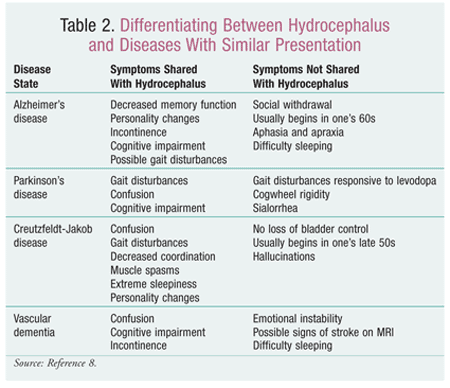
Since there are many conditions that could potentially cause symptoms suggestive of hydrocephalus, the most effective process for identification is to determine the symptoms, complete a differential diagnosis to rule out other causes (TABLE 2), and perform a CT scan or MRI of the brain to detect any ventriculomegaly.8
Treatment Options
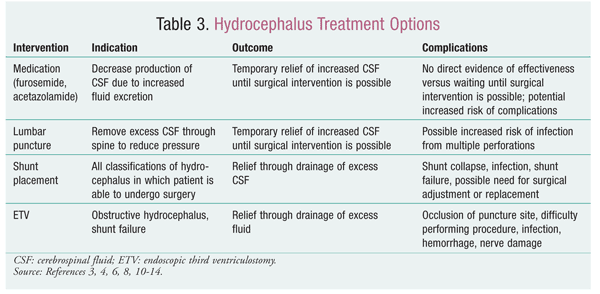
Although many causes of hydrocephalus exist, the number of treatments is limited. All successful, long-term treatments are surgical. There is little use for medication in hydrocephalus. In some acquired cases, as with tumors and infections, resolving the underlying condition will resolve the hydrocephalus, but most patients still require surgical intervention.6
There are generally two approaches to treating hydrocephalus. The most common treatment is the placement of a shunt.3 In use since the 1950s, this approach is considered the best treatment option in most cases. The other procedure, endoscopic third ventriculostomy (ETV), involves the surgical creation of an opening in the floor of the third ventricle to enable the passage of CSF.6,9
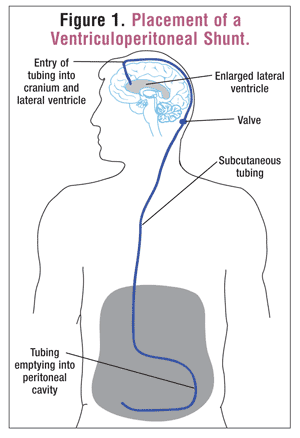
Shunt Placement: As noted above, the standard treatment for hydrocephalus is shunt placement. Shunts are usually placed in the lateral ventricle and can have one of three different drainage points. The most common drainage site is the peritoneum, which is connected to the shunt with subcutaneous tubing.3,8 This is known as a ventriculoperitoneal shunt. Two other types of shunts, ventriculopleural and ventriculoatrial, terminate in the pleural space and the internal jugular vein, respectively.3 The last type, the lumboperitoneal shunt, is placed in the lumbar intradural space.3 Shunt systems include a valve that controls the rate of drainage. The valve may have to be accessed surgically, or it may be placed so that adjustments can be made without further surgery (FIGURE 1).
With current standards and infection control, the postoperative mortality rate for shunt placement is less than 5%.10 Although shunt placement is considered the best treatment for hydrocephalus, it has several drawbacks. Shunt failure occurs at a rate of approximately 20% and can be caused by overdrainage, obstruction, or shunt collapse.8,11 Infection, which eventually can lead to sepsis, occurs at a rate of 5% to 15%.4 Antibiotic-infused shunt catheters appear to greatly reduce the rate of infection.4
ETV: As mentioned previously, ETV is an alternative treatment for hydrocephalus. It is indicated in patients with an obstruction that prevents CSF from draining between the third ventricle and the cortical subarachnoid spaces.9 In this procedure, the floor of the third ventricle is punctured, allowing CSF to flow into the cortical subarachnoid space.6,9 ETV is currently approved in the treatment of obstructive hydrocephalus and in patients who have had multiple shunt failures and replacements.6,9 ETV also has been shown to be effective for treating NPH.9 The procedure is difficult and requires that the third ventricle floor and the surrounding structures have dimensions appropriate for successful completion of the procedure.6 Imaging must be performed before surgery to determine that the anatomy of these structures is appropriate.6
One risk of ETV is piercing an unseen artery on the opposite side of the third ventricle floor, and infection can occur, although the rate is lower than that for shunts. There is also a risk of the drain becoming occluded, which would necessitate additional surgery. ETV may not be effective in neonates. The survival rate is extremely low for infants younger than 3 months, but reaches 64% around age 6 months. ETV generally is not performed in neonates if other options are available.10
Lumbar Puncture: A short-term option for the treatment of hydrocephalus is to perform periodic lumbar punctures. This is a temporary approach to reduce the amount of CSF until a long-term treatment can be performed.12 Lumbar puncture may be used to treat communicating hydrocephalus, although it is sufficient only for patients who are still able to absorb some CSF.12 In some cases, a drain may be placed so that continuous lumbar tapping is not necessary; however, there is a relatively high rate of infection with this approach compared with serial lumbar punctures.12 Lumbar puncture may be used in neonates, who have an extremely low surgical success rate with ETV.13
Noninvasive Treatments: At present, there are no significant noninvasive treatment alternatives. Some studies have shown that acetazolamide or furosemide may be acceptable for CSF fluid reduction, but these medications currently are used only on a temporary basis.14 Both drugs act to reduce the production of CSF by the choroid plexus.14 As with lumbar puncture, these agents are typically used in low-birthweight infants who will have a low success rate with shunt placement or ETV.13 There is no evidence that either of these medications increases survival rates, and a Cochrane review concluded that therapy with acetazolamide or furosemide is neither effective nor safe for treating posthemorrhagic ventricular dilatation in infants.14
TABLE 3 gives a brief summary of the different treatment options available for the treatment of hydrocephalus.3,4,6,8,10-14
Long-Term Prognosis
The treatment of hydrocephalus requires long-term care and lifelong follow-up. This is especially true for children and neonates whose hydrocephalus has a congenital cause. Even after appropriate placement of a shunt, annual neurologic visits are required.11 Complications, including shunt collapse, infection, and occlusions, can cause a rise in CSF pressure that may lead to symptom recurrence and the need for additional surgeries.3 Recognizing these signs is challenging because a number of other diseases have symptoms similar to those of hydrocephalus. Delaying medical treatment can be deadly, and all patients with a history of hydrocephalus need to be aware of pertinent symptoms and seek help immediately if they arise.
Children with hydrocephalus face developmental disorders as they age. Hydrocephalus patients have reduced motor function, a lower-than-average adult IQ, and decreased visual function; they also are at risk for developing epilepsy.11 The extent of the complications observed is dependent upon the type of hydrocephalus, but patients with epileptic seizures (approximately 30%) have the worst clinical outcomes and, compared with patients who did not develop seizures, are more likely to have an IQ lower than 90.15 About 60% of children with hydrocephalus are able to attend school (although many have difficulties), and approximately 40% of children will lead relatively normal lives.1,10,11
Conclusion
Hydrocephalus is a complicated condition to diagnose, and treatment options are limited. Current long-term treatment options are restricted to surgical interventions, including shunt placement and ETV. Even if the underlying disease state, such as an infection or tumor, is resolved, most patients will require surgical intervention and long-term follow-up.
REFERENCES
1. National Institute of Neurological Disorders and Stroke.
Hydrocephalus fact sheet.
www.ninds.nih.gov/disorders/hydrocephalus/detail_hydrocephalus.htm.
Accessed January 30, 2013.
2. Mitropoulos IF, Hermsen ED, Schafer JA, Rotschafer JC. Central
nervous system infections. In: DiPiro JT, Talbert RL, Yee GC, et al,
eds. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw-Hill Medical; 2008:1744.
3. Kandasamy J, Jenkinson MD, Mallucci CL. Contemporary management and recent advances in paediatric hydrocephalus. BMJ. 2011;343:146-151.
4. Parker SL, Attenello FJ, Sciubba DM, et al. Comparison of shunt
infection incidence in high-risk subgroups receiving
antibiotic-impregnated versus standard shunts. Childs Nerv Syst. 2009;25:77-83.
5. Vinchon M, Baroncini M, Delestret I. Adult outcome of pediatric hydrocephalus. Childs Nerv Syst. 2012;28:847-854.
6. Yadav Y, Parihar V, Pande S, et al. Endoscopic third ventriculostomy. J Neurosci Rural Pract. 2012;3:163-173.
7. Oi S. Classification of hydrocephalus: critical analysis of
classification categories and advantages of “Multi-categorical
Hydrocephalus Classification” (Mc HC). Childs Nerv Syst. 2011;27:1523-1533.
8. Neumiller J, Neumiller J, Gates B, et al. Normal pressure hydrocephalus. US Pharm. 2007;32(1):56-61.
9. Moorthy RK, Rajshekhar V. Endoscopic third ventriculostomy for
hydrocephalus: a review of indications, outcomes, and complications. Neurol India. 2011;59:848-854.
10. Hoppe-Hirsch E, Laroussinie F, Brunet L, et al. Late outcome of the surgical treatment of hydrocephalus. Childs Nerv Syst. 1998;14:97-99.
11. Vinchon M, Rekate H, Kulkarni AV. Pediatric hydrocephalus outcomes: a review. Fluids Barriers CNS. 2012;9:18.
12. Heep A, Engelskirchen R, Holschneider A, Groneck P. Primary
intervention for posthemorrhagic hydrocephalus in very low birthweight
infants by ventriculostomy. Childs Nerv Syst. 2001;17:47-51.
13. Kulkarni AV, Drake JM, Mallucci CL, et al. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr. 2009;155:254-259.
14. Whitelaw A, Kennedy CR, Brion LP. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database Syst Rev. 2001(2):CD002270.
15. Bourgeois M, Sainte-Rose C, Cinalli G, et al. Epilepsy in children with shunted hydrocephalus. J Neurosurg. 1999;90:274-281.
To comment on this article, contact rdavidson@uspharmacist.com.





