US Pharm. 2012;37(3):HS6-HS8.
Reye’s syndrome is a rare and potentially fatal condition affecting children and adolescents, initially described by Australian pathologist R.D. Reye in 1963.1 It is a metabolic noninflammatory encephalopathy associated with fatty degeneration of the liver.2 Reye’s syndrome is typically preceded by a viral illness, and generally presents with severe protracted vomiting, followed by encephalopathy that may progress to coma or death, or may spontaneously resolve.3 Residual neurocognitive effects may remain after recovery from Reye’s syndrome, although some children recover completely.
Historical Perspective
The initial report by R.D. Reye described 21 children who presented with severe vomiting, tachypnea, hypoglycemia, and elevated liver enzymes.1 They also experienced mental status changes and varying degrees of reduced consciousness, some progressing to coma. Of these 21 children, 17 died within the first 3 days of admission. Prior to this syndrome, each of the patients had experienced malaise, usually associated with an upper respiratory infection. In this report, Reye notes that the etiology may not be identical in all of these 21 cases.1
In the mid 1960s, after the publication of Reye’s report, physicians and researchers around the world worked to identify a cause for Reye’s syndrome. As early as 1965, it was suggested that the syndrome could be caused by hypersensitivity to salicylates.4 This view was generally accepted by the late 1980s.5 In 1986, the FDA placed a warning on all OTC aspirin-containing products, and a warning remains on aspirin products today.3 It reads: “Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.” However, the link between aspirin and Reye’s syndrome has not been proven, and this link is still challenged and debated today.
In 1977, 454 cases of Reye’s syndrome were reported in the United States. Of the 373 cases with follow-up, 42% of these patients died, and 11% survived with residual neurologic damage.6 Incidence was increased with viral epidemics, especially influenza B and varicella. Reye’s syndrome cases in the U.S. numbered 555 in 1980, but they have fallen drastically. From 1994 until 1997, identified cases in the U.S. were fewer than two per year.7 The mean mortality rate of Reye’s syndrome is approximately 40% and appears to be higher in males than in females.8
Decreasing Incidence
There are several proposed causes for the drastically reduced rate of Reye’s syndrome cases. Because of efforts to educate the public regarding the potential danger of using aspirin in children and adolescents, aspirin use in this population has been largely abandoned, except for several clinical scenarios in which aspirin is significantly beneficial, such as Kawasaki disease. Another reason may be improvement in the recognition and diagnosis of various metabolic diseases and inborn errors of metabolism, which may present with symptoms similar to Reye’s syndrome. In fact, some of the cases originally diagnosed as Reye’s syndrome were later found to be patients with metabolic disorders. In 2008, a report by Gosalakkal and Kamoji describes a 14-year-old girl presenting with symptoms very similar to Reye’s syndrome.9 It was determined that the patient actually had long-chain acyl-coenzyme A (CoA) dehydrogenase deficiency, a metabolic disorder. An additional explanation that has been proposed to explain the changing rates is viral mutation over time.10
Case Reports
Although the incidence of Reye’s syndrome is certainly dropping, there are still reports in the literature. McGovern et al report two cases of Reye’s syndrome occurring in 1999 in a 12-year-old boy and a 9-month-old boy.11 Both children were given aspirin, and metabolic disorders were ruled out. Bhutta et al reported on a 3-year-old boy in 1999 with recent history of fever and upper respiratory infection who presented with vomiting and mental status changes.12 This patient had been given aspirin during his febrile illness and also promethazine for his vomiting. Liver biopsy was consistent with Reye’s syndrome, and severe cerebral edema resulted in herniation. Autopsy results supported the diagnosis of Reye’s syndrome. Chow and Cherry report a 10-year-old girl presenting in 2002 with a recent history of sore throat and upper respiratory infection, for which she had taken aspirin.6 Three days following her aspirin therapy, she experienced progressive vomiting and dehydration, followed by confusion and combativeness. Once admitted, she became obtunded and apneic and was therefore intubated. Symptoms and biopsies were consistent with Reye’s syndrome. After a few days, the patient was declared brain dead.
A case of Reye’s syndrome associated with H3N2 influenza and salicylate intake was reported by Ninove et al.13 The patient was a 12-year-old boy who presented in early 2009 with a recent history of upper respiratory tract infection, later found to be influenza A (H3N2), and fever. He had been treated with aspirin for this illness. Two days later, he was admitted with neurologic distress and gastrointestinal (GI) disease and hematemesis. He also had hypoglycemia and liver dysfunction. Soon after, he experienced confusion and drowsiness, and later cerebral edema. This patient died 2 days after admission.
Pathophysiology and Risk Factors
The pathophysiology of Reye’s syndrome is thought to involve mitochondrial injury, which inhibits oxidative phosphorylation and fatty-acid beta-oxidation. High concentrations of ammonia may accumulate due to the decrease in the activity of the mitochondria. On biopsy, the liver will appear fatty, and changes to the appearance of the mitochondria are often evident. A brain biopsy will show similar findings, along with edema. The proposed mechanism for aspirin contributing to the development of Reye’s syndrome is related to mitochondrial damage that can be caused by salicylates, which may be intensified during viral illness by endotoxins and cytokines.14
Causation of Reye’s syndrome remains unclear and is a topic of debate.14 There are many published case-control studies that support a strong association between aspirin and Reye’s syndrome. One study found a dose-response effect as well.15 A total dose of less than 45 mg/kg of aspirin was found to increase the risk of Reye’s syndrome 20-fold, and the authors concluded that any amount of aspirin is unsafe in a child with a viral infection, regardless of the dose. Many authors point to the radically decreasing rates of Reye’s syndrome that occurred with the cessation of aspirin use in children as evidence of a causative link as well. Since it is now recognized that several metabolic disorders can cause symptoms that are very similar to Reye’s syndrome, many hypothesize that reported Reye’s syndrome cases represented a variety of disorders. These disorders likely include metabolic disorders that were not diagnosed at the time, and may also include idiopathic Reye’s syndrome in which aspirin was, in fact, a contributing factor.16
Others, however, claim that the association between aspirin and Reye’s syndrome does not necessarily prove a causal relationship. Reye’s syndrome was first described in 1963, but aspirin was used in children long before the 1960s. In addition, aspirin was not universally used in all children who developed Reye’s syndrome, and there were some control patients without the syndrome who had taken aspirin. Skeptics of this link further highlight that these case studies are retrospective, and recall bias can be an issue with this type of study design. They also point out that at the time most of these case studies were done, parents may not have distinguished between acetaminophen and aspirin.10 Salicylate serum concentrations were usually not measured in the patients studied, and biopsy confirmation of the Reye’s syndrome diagnosis was also frequently not obtained.10 As such, some authors have questioned whether aspirin does, in fact, need to be avoided by children.17
Several infectious agents have been correlated with Reye’s syndrome and Reye-like syndrome. Viral agents include influenza A and B, varicella, parainfluenza, measles, adenoviruses, coxsackie viruses, cytomegalovirus, Epstein–Barr virus, HIV, hepatitis A and B, and rotavirus.18 Some bacterial agents may play a role as well, including Mycoplasma, Chlamydia, Shigella, and Salmonella.18
Of note, an association has also been observed between Reye’s syndrome and several other medications and toxins, including hypoglycin from the ackee fruit, aflatoxin, paint-thinner, acetaminophen, valproic acid, outdated tetracycline, zidovudine, and didanosine.19 The relationship of these agents to Reye’s syndrome is not as strong of an association as that of aspirin and the syndrome. A possible role of antiemetics was suggested in the 1970s because at that time, as many as 71% of Reye’s syndrome cases were associated with phenothiazine use early in the course of the disease.19 This hypothesis was discarded based on the results of epidemiologic studies in the 1980s, but some authors and practitioners argue that this relationship may, in fact, be significant.19
Diagnosis
Because no test is specific for Reye’s syndrome, it is a diagnosis of exclusion. A high index of suspicion must be present in a patient with vomiting and mental status changes. Diagnostic criteria from the CDC are listed in TABLE 1.20
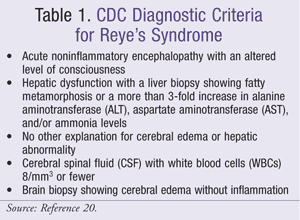
Diseases and conditions that resemble Reye’s syndrome must be ruled out. These conditions include metabolic disorders such as defects in fatty acid oxidation (including acyl-CoA dehydrogenase deficiencies), disorders of oxidative phosphorylation, urea-cycle defects, disorders of carbohydrate metabolism, other metabolic disorders and errors of inborn metabolism, CNS infection or meningitis, and drug or toxic ingestion. Disorders causing presentation with acute liver failure should also be ruled out.
Clinical Presentation
Reye’s syndrome is a biphasic condition that typically occurs in a child who is otherwise healthy. It begins as a prodromal febrile illness that is likely viral in nature, such as an upper respiratory infection or varicella, or possibly rotavirus.21 After this illness resolves, the child will recover for 3 to 5 days.18 Following this period is sudden onset of protracted vomiting along with a degree of mental status changes that will vary with severity of disease. Patients will often present with some degree of hepatomegaly and hepatic dysfunction due to fatty degeneration of the liver, but they will not be icteric. There also may be an elevation of intracranial pressure.
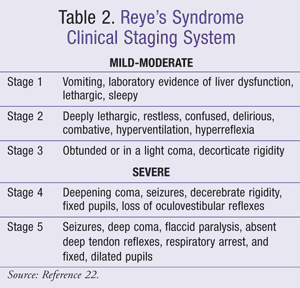
The severity of Reye’s syndrome can be classified with the clinical staging system (TABLE 2).22 Stages 1 through 3 are considered to represent mild-to-moderate disease, while stages 4 and 5 of the illness represent severe Reye’s syndrome.
Treatment
There is no specific treatment for Reye’s syndrome, and therefore the appropriate treatment strategy is to provide supportive care. Early diagnosis is important, and other diseases causing similar symptoms must be ruled out. Hypoglycemia can be treated by administering fluids such as 10% dextrose, thus providing glucose to the patient. Coagulopathies may be managed with blood transfusions, and intubation may be necessary to provide adequate oxygenation. Intracranial pressure must be monitored and controlled in patients with cerebral edema. Management of cerebral edema may involve fluid restriction, diuresis, and corticosteroids. Pentobarbital has been given to patients to decrease cerebral metabolic demands.23 Therapies to reduce hyperammonemia may also be indicated. In patients who develop seizures, antiepileptic medications may be necessary.
Conclusion
Reye’s syndrome is a serious condition of children and adolescents that is rarely seen today. Aspirin is considered to be the most significant causative agent, although this association is often challenged. Pharmacists can play a role in ensuring the appropriate use of aspirin in the pediatric population. Despite the controversy regarding the relationship of aspirin to Reye’s syndrome, this is a condition with significant morbidity and mortality and therefore it is prudent to continue to limit the use of aspirin in children. A risk-benefit assessment must be done on a patient-by-patient basis. While the benefit likely outweighs the risk in patients with disorders such as Kawasaki disease, the pharmacist can play a role in ensuring that these patients receive their influenza vaccination to decrease their chance of viral illness during their aspirin therapy. In otherwise healthy children, the use of aspirin as an antipyretic or anti-inflammatory agent is not recommended because the benefit likely does not outweigh the risk. In this circumstance, the pharmacist can play an important role in recommending an alternative agent, such as ibuprofen or acetaminophen.
REFERENCES
1. Reye RD, Morgan G, Baral J. Encephalopathy and fatty degeneration of the viscera: a disease entity in childhood. Lancet. 1963;2:749-752.
2. Schrör K. Aspirin and Reye syndrome: a review of the evidence. Paediatr Drugs. 2007;9:195-204.
3. Fitzgerald DA. Aspirin and Reye syndrome. Paediatr Drugs. 2007;9:205-206.
4. Giles HM. Encephalopathy and fatty degeneration of the liver. Lancet. 1965;1:1075.
5. Clark I, Whitten R, Molyneux M, et al. Salicylates, nitric oxide, malaria, and Reye’s syndrome. Lancet. 2001;357:625-627.
6. Chow EL, Cherry JD. Reassessing Reye syndrome. Arch Pediatr Adolesc Med. 2003;157:1241-1242.
7. Belay ED, Bresee JS, Holman RC, et al. Reye’s syndrome in the United States from 1981 through 1997. N Engl J Med. 1999;340:1377-1382.
8. Luscombe FA, Monto AS, Baublis JV. Mortality due to Reye’s syndrome in Michigan: distribution and longitudinal trends. J Infect Dis. 1980;142:363-371.
9. Gosalakkal JA, Kamoji V. Reye syndrome and Reye-like syndrome. Pediatr Neurol. 2008;39:198-200.
10. Orlowski JP, Hanhan UA, Fiallos MR. Is aspirin a cause of Reye’s syndrome? A case against. Drug Saf. 2002;25:225-231.
11. McGovern MC, Glasgow JF, Stewart MC. Lesosn of the week: Reye’s syndrome and aspirin: lest we forget. BMJ. 2001;322:1591-1592.
12. Bhutta AT, Van Savell H, Schexnayder SM. Reye’s syndrome: down but not out. South Med J. 2003;96:43-45.
13. Ninove L, Daniel L, Gallou J, et al. Fatal case of
Reye’s syndrome associated with H3N2 influenza virus infection and
salicylate intake in a 12-year-old patient. Clin Microbiol Infect. 2011;17:95-97.
14. Glasgow JF, Middleton B. Reye syndrome—insights on causation and prognosis. Arch Dis Child. 2001;85:351-353.
15. Forsyth BW, Horwitz RI, Acampora D, et al. New
epidemiologic evidence confirming that bias does not explain the
aspirin/Reye’s syndrome association. JAMA. 1989;261:2517-2524.
16. Glasgow JF. Reye’s syndrome: the case for a causal link with aspirin. Drug Saf. 2006;29:1111-1121.
17. Langford NJ. Aspirin and Reye’s syndrome: is the response appropriate? J Clin Pharm Ther. 2002;27:157-160.
18. Pugliese A, Beltramo T, Torre D. Reye’s and Reye’s-like syndromes. Cell Biochem Funct. 2008;26:741-746.
19. Casteels-Van Daele M, Van Geet C, Wouters C, et al.
Reye syndrome revisited: a descriptive term covering a group of
heterogeneous disorders. Eur J Pediatr. 2000;159:641-648.
20. CDC. National Reye syndrome surveillance—United States, 1982 and 1983. MMWR Morb Mortal Wkly Rep. 1984;33:41-42.
21. Devulapalli CS. Rotavirus gastroenteritis possibly causing Reye syndrome. Acta Pediatr. 2000;89:613-619.
22. Lovejoy FH, Smith AL, Bresnan MJ, et al. Clinical staging in Reye syndrome. Am J Dis Child. 1974;128:36-41.
23. Balistreri WF. Reye syndrome and “Reye-like” diseases. In: Behrman RE, Kliegman RM, Jenson HB, ed. Nelson Textbook of Pediatrics. 16th ed. Philadelphia: WB Saunders Company; 2000:1215-1216.
To comment on this article, contact rdavidson@uspharmacist.com.





