US Pharm. 2011;36(5):HS4-HS8.
An estimated 50 million Americans currently suffer from chronic pain, along with another 25 million who suffer from acute pain.1 Each year, nearly half of all Americans present to their physician with a chief complaint of pain.2 The mainstay of treatment of moderate-to-severe pain is opioid analgesics, with the addition of other analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) and other adjuvant therapies, including tricyclic antidepressants (TCAs), anticonvulsants, and topical anesthetics as necessary.3,4 However, more than four out of 10 people with moderate-to-severe pain do not get relief, according to an American Pain Society (APS) survey conducted in 1999.5 Of those who do not get pain relief, some may be experiencing pain that is not responsive to opioids or other drug therapies despite appropriate use of these agents, as in opioid resistance and neuropathic pain.
Opioid Resistance and Neuropathic Pain
Opioid resistance is defined as unresponsiveness to IV morphine sulfate of at least 100 mg per hour (or equivalent dosing of another opioid), consistently high pain ratings, and unrelieved pain even after the opioid dose is doubled. Opioid resistance has been found in a multitude of disease states including cancer, chronic pain, neuropathy, complex regional pain syndrome, postherpetic neuralgia, and pancreatitis.6 Neuropathic pain results from injury to peripheral or central nerves and is commonly treated with agents such as TCAs and anticonvulsants. Unfortunately, a majority of patients do not experience significant relief with these agents.7,8 In both opioid resistance and neuropathic pain, N-methyl-d-aspartate (NMDA) antagonists may be an option.
NMDA Receptor Antagonists
NMDA is a receptor for the excitatory neurotransmitter glutamate, which is released with noxious peripheral stimuli.7,9 The activation of NMDA receptors has been associated with hyperalgesia, neuropathic pain, and reduced functionality of opioid receptors. Hyperalgesia and neuropathic pain are a result of increased spinal neuron sensitization, leading to a heightened level of pain.7,9,10 The reduced function of opioid receptors is caused by a decrease in the opioid receptor’s sensitivity. This decreased sensitivity, in turn, translates to opioid tolerance as patients will require higher doses of opioids to achieve the same therapeutic effects.9 Therefore, NMDA antagonists may have a role in these areas of pain management.10
There are several NMDA receptor antagonists available: ketamine, methadone, memantine, amantadine, and dextromethorphan (TABLE 1).11,12 They each differ in their level of activity on the NMDA receptor. Ketamine is a strong NMDA antagonist, whereas the others are weaker NMDA receptor blockers.13 Severity and frequency of side effects depend on affinity for the NMDA receptor. In adults, adverse effects of NMDA antagonists are mainly central nervous system (CNS) side effects including hallucinations, lightheadedness, dizziness, fatigue, headache, out-of-body sensation, nightmares, and sensory changes. Since ketamine is a strong NMDA antagonist, it is less tolerable than the other antagonists due to a higher incidence of side effects, in particular hallucinations and a dissociative mental state.7
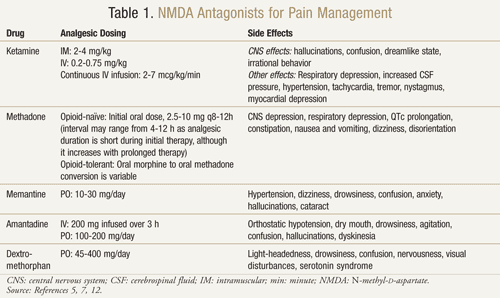
Ketamine: Ketamine has proved beneficial in multiple pain settings. In a clinical trial, the addition of low dose IV ketamine to opioids versus opioids alone postoperatively after major abdominal surgery produced better analgesia, less sedation, and decreased need of morphine or physician intervention to manage pain.14 A randomized, double-blind, crossover, placebo-controlled trial was also conducted to confirm previous results that suggested ketamine was effective in cancer patients who were resistant to morphine.15 Each of the 10 enrolled patients received ketamine at a dose of 0.25 mg/kg, 0.50 mg/kg, and placebo on 3 separate days at least 2 days apart in addition to their morphine. The results showed that ketamine significantly reduced pain at both doses compared to placebo. Patients receiving 0.5 mg/kg had a better analgesic effect than patients on 0.25 mg/kg (P <.05). Significant adverse effects occurred in four patients who experienced hallucinations and two patients who experienced an unpleasant sensation to which they referred as “empty head.” Patients received diazepam 1 mg for a successful reversal of these CNS adverse effects. Patients experienced significant drowsiness with both doses of ketamine, although it was most pronounced at the 0.5 mg/kg dose (P <.05).15
In a prospective, multicenter, unblinded, open-label trial of 39 patients, “burst” ketamine infusion was shown to have a significant effect on cancer-related pain in patients who were either opioid resistant even with the addition of adjuvant analgesics or intolerant to the adverse effects of opioids.16 Burst ketamine was defined as a short-duration (3-5 day) subcutaneous infusion, beginning at an initial dose of 100 mg/24 h, then escalating to 300 mg/24 h and 500 mg/24 h in a stepwise fashion if the patient’s pain was persistent without intolerable side effects. Pain relief was analyzed by type of pain (somatic, visceral, or neuropathic). Those patients with more than one pain type had each of their pains analyzed separately. Of 43 pains treated in 39 patients, 29 (67%) showed at least a 50% decrease on the verbal rating scale (0 = no pain, 10 = worst possible pain) supported by a significant reduction in opioid use over 24 hours and/or improved mobility or functional status. Twelve patients reported CNS adverse effects including feeling “spaced out,” hallucinations, drowsiness, and dizziness.16
Methadone: Methadone is another NMDA antagonist that has been studied in opioid resistance and neuropathic pain. It has been shown to be a good option to use as a replacement opioid in patients who are poorly controlled or experience dose-limiting adverse effects while on other opioids.17 In 80% of cancer patients with uncontrolled pain or significant side effects, methadone has demonstrated a reduction of pain and adverse effects after a switch from morphine to methadone.18
Methadone has also demonstrated effectiveness in patients with refractory neuropathic pain. In a double-blind, randomized, controlled, crossover trial conducted in 18 patients who did not respond to traditional analgesic regimens for their chronic neuropathic pain, 10 mg twice daily oral methadone showed statistically significant pain relief in maximum pain intensity (P = .013), average pain intensity (P = .020), and relief of pain (P = .015) when compared to placebo.19 Methadone at 5 mg twice daily also showed analgesic improvement in maximum pain intensity and pain relief; however, it did not reach statistical significance. Six patients withdrew from the study due to adverse events including nausea, vomiting, dizziness, sweating, and disorientation with severe headaches. Others who completed the trial only reported having mild-to-moderate adverse effects.19
Gagnon et al conducted a trial of methadone in the treatment of neuropathic pain in 18 patients who either did not receive opioids for their pain or were receiving a daily dose of opioids no greater than an equivalent of 120 mg of oral morphine due to side effects that prevented further dose escalation.8 Methadone doses were started between 2 mg and 5 mg three times a day depending on age and titrated up to a stable dose based on clinical response and adverse effects. Methadone 2 mg every 4 to 6 hours was allowed for breakthrough pain as needed. The patients were followed for a median of 106 days (16 to 466 days). All patients experienced an improvement in their visual analog scale (VAS; 0-10 cm, where 0 = no pain and 10 = worst possible pain) pain scores with methadone treatment. The average pretreatment VAS ± standard deviation (SD) was 7.7 ± 1.5 cm and decreased to 1.4 ± 1.7 cm when on methadone (P <.0001). Nine out of 18 (50%) had no pain at all while they were on a stable dose of methadone. Of the 13 patients who had allodynia as part of their neuropathic pain, 9 (70%) showed complete resolution (no clinically detectable allodynia) and 4 (30%) had partial resolution (allodynia in <50% of the surface area). Of the 8 patients who had shooting pain, all 8 (100%) reported symptom control on a stable methadone dose. Side effects included mild drowsiness and nausea that was transient, along with constipation that was resolved with laxatives.8
Unfortunately, methadone is often challenging to use given its long and variable half-life of approximately 8 to 59 hours, required ECG monitoring for possible QTc prolongation, and many drug interactions with other QTc prolonging agents, as well as CYP3A4 and CYP2D6 inhibitors.12 In addition, opioid conversion is difficult as methadone increases in potency with increasing doses of morphine. Therefore, no single ratio for equianalgesic dosing can be found between morphine and methadone.17
Memantine: Other NMDA receptor antagonists such as memantine, amantadine, and dextromethorphan have shown mixed results in neuropathic pain.20 Memantine has a safe side-effect profile and rapid onset of action; however, in a randomized, double-blind, crossover study where memantine was administered to a group of 19 patients with chronic pain due to nerve injury after surgery, there was no difference in pain reduction with memantine versus placebo.21 In addition, a study with memantine in patients with HIV-associated sensory neuropathy did not show positive results.22
Amantadine: Amantadine is another drug that has shown mixed results in clinical trials. A double-blind, randomized, placebo-controlled trial was conducted in 15 cancer patients who had surgical neuropathic pain.23 In a randomized order, patients received a 200-mg infusion of amantadine or placebo 1 week apart from each other. Spontaneous and evoked pain were measured 48 hours before, during, and after treatment. On average, there was an 85% pain reduction with amantadine versus 45% with placebo (P = .009) at the end of the infusion. When comparing mean pain intensity 48 hours prior to and following treatment, amantadine had a 31% reduction in pain (P = .006), whereas the placebo showed an insignificant pain reduction of 6% (P = .40).23
In contrast to these positive results, Fukui et al conducted a study of amantadine in 19 patients who failed to respond to the conventional treatments for neuropathic pain, including anticonvulsants, antidepressants, and nerve blocks.24 The patients were started on oral amantadine 100 mg/day for 1 week and titrated to 200 mg/day. The results showed pain reduction in only 2 (10.5%) of the 19 patients. Adverse effects were experienced in 52.6% of the patients, including dry mouth, drowsiness, hallucinations, excitation, irritation, dizziness, dyskinesia, and loss of hair.24
Dextromethorphan: Commonly found in OTC cough medications, dextromethorphan has also been reviewed for its use in neuropathic pain. In a placebo-controlled, double-blind, randomized crossover study, 15 patients with neuropathic pain received 270 mg of dextromethorphan and placebo in random order separated by a 1-week washout period.25 The results showed a 30% reduction in pain after a single dextromethorphan dose compared to placebo. After 1.5 hours and 2.5 to 4 hours from time of medication, there was a statistically significant difference in pain reduction of dextromethorphan versus placebo (P <.05 and P <.002, respectively). Side effects included light-headedness, drowsiness, visual disturbances, and hot flushes; none were severe.25
As dextromethorphan is metabolized via CYP2D6 to the active metabolite dextrorphan, extensive versus poor metabolizers were also compared. Patients who were extensive metabolizers of dextromethorphan experienced better analgesia than poor metabolizers. It was concluded that dextromethorphan has potential in the treatment of neuropathic pain, but more extensive studies are needed to validate its use.25
Role of the Pharmacist
Pharmacists can impact patient care greatly in individuals who are not receiving adequate pain relief due to opioid resistance and neuropathic pain not adequately controlled by commonly used agents, by understanding the current role of NMDA antagonists. As methadone has currently shown a great deal of promise in this area, it is important that pharmacists understand how to facilitate the safe use of this agent in regard to proper dosing, drug interactions, and monitoring of adverse effects.
Conclusion
NMDA antagonists are a great venue to explore in the treatment of opioid-resistant and neuropathic pain. The NMDA antagonists that have currently been tested include ketamine, methadone, memantine, amantadine, and dextromethorphan. The clinical trials so far have demonstrated the value of ketamine and methadone in reduction of neuropathic pain and opioid-resistant pain. However, CNS adverse effects are a concern, especially with ketamine. Memantine, amantadine, and dextromethorphan are weaker NMDA antagonists with a safer toxicity profile but have not shown consistent benefit in these pain settings.12 More studies of NMDA-antagonists are needed to determine their best use in pain management as well as to effectively manage their side effects.
REFERENCES
1. Weiner K. Pain issues. Pain is an epidemic. American Academy of Pain Management. www.aist-pain.it/en/files/
2. Turk DC. Pain hurts—individuals, significant others, and society! APS Bulletin. 2006;16(1). www.ampainsoc.org/pub/
3. Chronic pain in America: roadblocks to relief. American Pain Society. 1999. http://ampainsoc.org/links/
4. Health Care Guidelines: Assessment and Management of Acute Pain. Institute For Clinical Systems Improvement. 6th ed. March 2008. www.icsi.org/pain_acute/pain__
April 8, 2011.
5. Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist.
6. McCormick WC, Schreiner RL. Diagnosis and treatment of opiate-resistant pain in advanced AIDS. West J Med. 2001;175:408-411.
7. Chizh BA, Headley PM. NMDA antagonists and neuropathic pain—multiple drug targets and multiple uses. Curr Pharm Des. 2005;11:2977-2994.
8. Gagnon B, Almahrezi A, Schreier G. Methadone in the treatment of neuropathic pain. Pain Res Manag. 2003;8:149-154.
9. Bennett GJ. Update on the neurophysiology of pain transmission and modulation: focus on the NMDA-receptor. J Pain Symptom Manage. 2000;19(suppl 1):S2-S6.
10. DuPen A, Shen D, Ersek M. Mechanisms of opioid-induced tolerance and hyperalgesia. Pain Manag Nursing. 2007;8:113-121.
11. Hewitt DJ. The use of NMDA-receptor antagonists in the treatment of chronic pain. Clin J Pain. 2004;9:571-591. 2000;16(suppl 2):S73-S79. http://opioids.com/nmda/nmda.
12. Lexicomp Online. www.lexi.com. Accessed April 12, 2011.
13. Feinberg S, Provenzano D. ACPA Consumer Guide to Pain Medication & Treatment. Rocklin, CA: American Chronic Pain Association Inc; 2010. www.theacpa.org/documents/
14. Bhavani-Shankar K, Jasmeet SO. Management of postoperative pain. UptoDate. May 28, 2010. www.uptodate.com. Accessed October 4, 2011.
15. Mercadante S, Arcuri E, Tirelli W, Casuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double-blind, crossover, double-dose study. J Pain Symptom Manage. 2000;20:246-252.
16. Jackson K, Ashby M, Martin P, et al. “Burst” ketamine for refractory cancer pain: an open-label audit of 39 Patients. J Pain Symptom Manage. 2001;22;831-842.
17. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
18. Mecadante S, Casuccio A, Fulfaro F, et al. Switching from morphine to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol. 2001;19:2898-2904.
19. Morley JS, Bridson J, Nash TP, et al. Low-dose methadone has an analgesic effect in neuropathic pain: a double-blind randomized controlled crossover trial. Palliat Med. 2003;17:576-587.
20. Portenoy RK, Ahmed E, Keilson YY. Cancer pain management: adjuvant analgesics. UptoDate. May 28, 2010. www.uptodate.com. Accessed October 4, 2011.
21. Nikolajsen L, Gottrup H, Kristensen AG, Jensen TS. Memantine (a N-methyl-d-aspartate receptor antagonist) in the treatment of neuropathic pain after amputation or surgery: a randomized, double-blinded, cross-over study. Anesth Analg. 2000;91:960-966.
22. Schifitto G, Yiannoutsos CT, Simpson DM, et al. A placebo-controlled study of memantine for the treatment of human immunodeficiency virus-associated sensory neuropathy. J Neurovirol. 2006;12:328-331.
23. Pud D, Eisenberg E, Spitzer A, et al. The NMDA receptor antagonist amantadine reduces surgical neuropathic pain in cancer patients: a double blind, randomized, placebo controlled trial. Pain. 1998;75:349-354.
24. Fukui S, Komoda Y, Nosaka S. Clinical application of amantadine, an NMDA antagonist, for neuropathic pain. J Anesth. 2001;15:179-181.
25. Carlsson KC, Hoem NO, Moberg ER, Mathisen LC. Analgesic effect of dextrometh-orphanin neuropathic pain. Acta Anaesthesiol Scand. 2004;48:328-336.
To comment on this article, contact rdavidson@uspharmacist.com.





